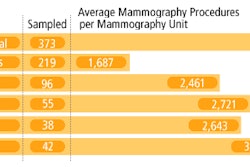
Fischer Imaging has received regulatory approval to market its full-field digital mammography (FFDM) system in the European Union, and has received two orders for the system, called SenoScan.
The CE Mark approval gives Denver-based Fischer the right to market SenoScan in the European market. The U.S. Food and Drug Administration in September gave the vendor premarket approval for sale of the system.
Fischer has received orders for its FFDM system from Falun Central Hospital in Sweden and from the Rene Huguenin Center in St. Cloud, France, near Paris.
By AuntMinnie.com staff writers
November 28, 2001
Related Reading
FFDM gains legitimacy as vendors clear regulatory hurdles, November 25, 2001
Is full-field digital mammography worth its weight in gold?, October 22, 2001
Fischer delivers first commercial SenoScan, October 3, 2001
Fischer joins short list of FDA-approved digital mammography vendors, September 26, 2001
Copyright © 2001 AuntMinnie.com