Pleasanton, CA-based Sanarus will highlight two products at this year's RSNA show: its Cassi Rotational Core Biopsy Device and Visica Treatment System. Both offerings were cleared by the FDA in 2004, and use the company's proprietary cryo technology.
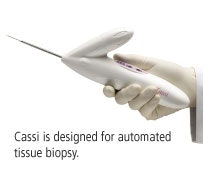
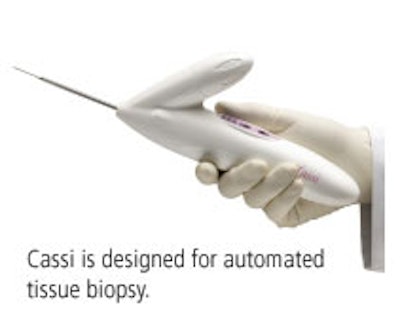
Cassi is a handheld, large-core biopsy device. Fully automated, it incorporates Sanarus's Stick-Freeze technology, which immobilizes the lesion for precise sampling of large tissue samples with fewer passes. Cassi is also disposable.
Visica is an in-office, ultrasound-guided, minimally invasive procedure that uses cryoablation technology to destroy fibroadenomas.
By Kate Madden Yee
AuntMinnie.com contributing writer
November 7, 2005
Copyright © 2005 AuntMinnie.com