Recently, the government-sponsored Agency for Healthcare Research and Quality (AHRQ) issued a report entitled "Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities."
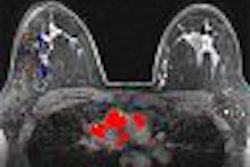
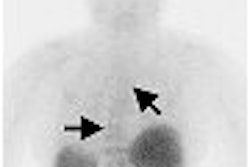
A literature review and analysis was undertaken to assess the role of PET, scintimammography, MRI, and ultrasound in breast imaging. The goal of this investigation was to "help patients, policymakers, and clinicians determine whether these noninvasive tests are sufficiently accurate to be appropriate for evaluation of women with an abnormal mammogram or exam finding."
The authors reviewed a total of 69 publications, nine on PET imaging, 45 on scintimammography, 19 on MRI, and eight on ultrasound. They looked at sensitivity, specificity, positive, and negative predictive value of the respective tests and concluded that none of the tests was sufficiently accurate to replace biopsy of suspicious abnormalities.
The Commission on Breast Imaging of the American College of Radiology (ACR) finds this report to be disturbing for various reasons. First, there seems to be a fundamental lack of understanding on the part of the authors as to the basic principles of breast imaging. The authors also demonstrated confusion as to what constitutes an "abnormal mammogram," and failed to recognize the difference between a screening mammogram with abnormal results and a mammogram with suspicious findings.
A woman without signs or symptoms related to the breast will have a screening mammogram. The purpose of this exam is to detect any areas that deserve further evaluation. If this exam is negative and nothing of concern is perceived, the woman returns in a year for another screening mammogram, as long as she remains symptom-free for breast disease.
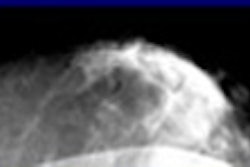
Should an area be detected on the screening mammogram, the woman is recalled and a diagnostic evaluation is performed. The purpose of the diagnostic exam is to validate the 3D presence of the questioned finding and to establish a management plan. An extremely important part of the diagnostic breast imaging work-up is ultrasound. Ultrasound has a role in both validation of findings suspected from the screening study and as an indispensable aid to separate cysts (100% benign when certain ultrasound criteria are met) from other findings.
The report shows no appreciation of the clinical context in which the studied tests are used. While ultrasound is commonly and frequently employed to evaluate an abnormal screening mammogram, PET, scintimammography, and MRI are rarely, if ever, used for that purpose in actual clinical practice.
In addition, the AHRQ report evaluated only eight ultrasound papers, only three of which dealt with nonpalpable findings and one of which evaluated a type of ultrasound that is not in widespread use (3D ultrasound).
The criteria for inclusion into this study excluded many pertinent and well-designed ultrasound papers that more appropriately address the question of the utility of ultrasound as a diagnostic test. In fact, in a review of papers designed specifically to investigate the utility of ultrasound in combination with mammography for evaluating palpable masses, Berg reported an overall negative predictive value of 98.6% (Berg WA, Breast Imaging in Oncology: An Evidence-Based Approach, Springer, New York City, 2006, pp. 381-391).
Another problem with the AHRQ report is that the conclusion that none of the tests is accurate enough to obviate the need for biopsy of a suspicious finding is already well-known in the breast imaging community and is the current standard of practice. No competent breast imager would recommend against biopsy of a mammographically or clinically suspicious finding because of a negative ancillary test, and indeed, these tests are not used in actual practice to dissuade biopsy of suspicious lesions.
Therefore, this report, which obviously required a substantial effort and expenditure of time and resources, is not clinically relevant to those practicing breast imaging, and only serves as a potential source of confusion to patients, policy makers, and clinicians who may be left with the impression that these tests are being used inappropriately.
It is unfortunate that the ACR was not consulted on this study before it was undertaken nor asked to comment upon it before its release. Without adequate input by those actually involved in the field being studied, reports such as this have no clinical relevance and have the potential of being misleading, counterproductive, and a waste of valuable resources.