CT laser mammography (CTLM) system developer Imaging Diagnostic Systems (IDSI) of Plantation, FL, has signed the 10th U.S. site to participate in its CTLM clinical trial.
Some sites have begun to collect patient data necessary to submit a Premarket Approval (PMA) application to the U.S. Food and Drug Administration. To date, international clinical sites have performed more than 13,000 CTLM exams, according to the company, but only U.S. data will be used for the PMA application.
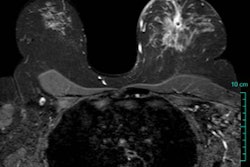
The CTLM system uses patented continuous wave laser technology and computer algorithms to create 3D breast images. The system is designed to gather information about blood distribution in the breast to detect angiogenesis, which usually accompanies tumor growth, and the images can be analyzed in tandem with standard mammography, according to the company.
Related Reading
IDSI sells HQ for $4.4 million in leaseback deal, September 19, 2007
IDSI sends CTLM unit to Malaysia, September 5, 2007
Study: IDS' CTLM aids in dense-breast imaging, June 26, 2007
IDSI drops China distributor, April 11, 2007
Hansen re-ups with IDSI, January 23, 2007
Copyright © 2007 AuntMinnie.com