One of the great things about full-field digital mammography (FFDM) is the ability to manipulate images in ways not possible with hard-copy analog film. But one image manipulation tool -- magnification mode -- can produce inaccurate measurements of lesion size when hardware and software from different vendors are paired.
That's according to a recent paper by Colorado researchers, who found that some combinations of FFDM systems and image review workstations produced discrepancies in lesion measurements that could lead to over- and underestimation of lesion sizes. In some cases, the inaccurate measurements were as much as 100% different from actual lesion size.
The problem appears to be particularly prevalent when FFDM units are used with display software from different manufacturers, and when FFDM units are used in different magnification modes. The discrepancies could make accurate preoperative cancer staging and treatment planning more difficult, according to the study, which was published in the American Journal of Roentgenology (January 2010, Volume 194:1, pp. W115-W118).
The problem apparently has escaped the attention of the U.S. Food and Drug Administration (FDA), according to Dr. Jean Paquelet of Advanced Medical Imaging Consultants in Fort Collins, CO, and R. Edward Hendrick, Ph.D., clinical professor of radiology at the University of Colorado in Denver.
"The FDA regulates every aspect of mammography," Hendrick told AuntMinnie.com. "But the incompatibility of different acquisition and display systems has slipped through the cracks."
Inaccurate measurement
Paquelet's and Hendrick's research was sparked by clinical experience, when inaccurate measurements were produced on a study of a 40-year-old woman. FFDM images were acquired with an Instrumentarium Performa mammography unit (GE Healthcare, Chalfont St. Giles, U.K.) using a computed radiography (CR) image receptor and reader (ClearView, Fujifilm Medical Systems USA, Stamford, CT). Images were reviewed on Fuji Synapse PACS software.
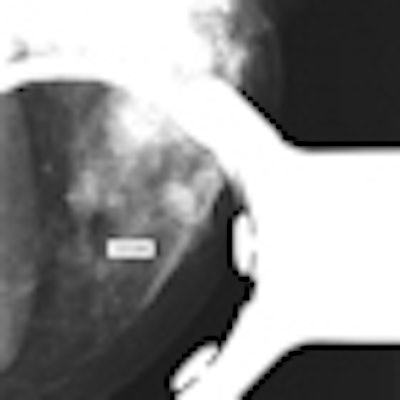
The researchers found that the electronic calipers on the PACS review workstation measured the lesion at 1.9 cm in 1.6X magnification mode. However, the same mass measured 1.0 cm on ultrasound and 0.9 cm on breast MR images, and pathologic evaluation revealed a 0.9-cm invasive ductal cancer.
"The discrepancy between mammographically determined cancer size and the cancer size measured on ultrasound, MRI, and the lumpectomy specimen shown at tumor board caused confusion concerning cancer staging and treatment planning," Paquelet and Hendrick wrote.
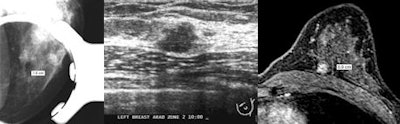 |
| Images demonstrate discrepancies in lesion measurements across different imaging modalities. At left, FFDM image at 1.6X magnification shows PACS software caliper tool indicating lesion size of 1.9 cm. In center, ultrasound of same lesion shows lesion size of 1.0 cm. At right, breast MR image of same lesion indicates size is 0.9 cm. Images courtesy of the American Roentgen Ray Society. |
To further study the phenomenon, they decided to examine the performance of three pairs of FFDM/workstation combinations, all of which individually have been cleared by the FDA and are commercially available. They tested the systems with an American College of Radiology (ACR) mammography phantom consisting of a 1-cm acrylic disk.
Digital mammograms were acquired in contact, or nonmagnification, mode and in magnification mode with each hardware/software combination. Electronic calipers were used to measure the size of the disk in each mode.
FFDM hardware/software combinations
|
With the system X combination (GE/Fuji), the magnification mode information was not passed from the mammography acquisition system to the CR processor or PACS, overestimating distances measured on magnified images by a factor equal to the magnification factor. The 1-cm acrylic disk measured 1.05 cm in nonmagnified mode, but incorrectly measured 1.64 cm at the 1.6X magnification level.
In the system Y combination (GE/Philips), magnification factors were passed from the FFDM unit to the PACS, but because of software incompatibilities, this factor was accounted for twice, registering a smaller lesion size for higher magnification factors. The 1-cm disk measured 0.99 cm in nonmagnified mode, but incorrectly measured 0.66 cm at the 1.5X magnification mode and 0.56 cm at the 1.8X magnification mode. When a GE acquisition display was used to view images acquired on the GE FFDM unit, all images showed the acrylic disk to measure 1 cm.
The system Z combination (Hologic/McKesson) gave accurate measurements of approximately 1 cm in both contact and 1.6X magnification modes.
Problems with mix and match?
The study highlights the problems that can occur when hardware and software from different manufactures are combined -- problems that many clinical users may not know exist.
"For sites using several different PACS, third-party, or manufacturer-provided review workstations for mammography interpretation, distinctly different distance measurement results could occur for geometrically magnified images displayed on different review workstations," the authors wrote.
One way to identify lesion measurement errors inherent in the system could be to include a radiographically visible ruler at the plane of the image receptor or compression paddle in every digital image, including magnification images. But this may not be practical, as the ruler itself could obscure breast tissue or interfere with the automatic exposure control function of the device, Paquelet and Hendrick wrote.
"Radiologists should be able to assume that lesion size is correctly indicated, whether the image is taken in contact or magnification mode," Hendrick told AuntMinnie.com. "But that's not necessarily the case. Our study demonstrates that in digital mammography, lesion size can be accurate in contact mode but not in magnification views. And that can be a big problem in terms of preoperative clinical staging of cancers and counseling of patients based on imaging findings."
In any case, distance measurement accuracy should be tested in contact mode and in each magnification mode for each digital acquisition and display combination, a responsibility that lands firmly in the purview of the medical physicist and should be included in routine quality control testing, according to Hendrick.
"The FDA is sometimes concerned about the wrong things," Hendrick said. "They focus on paperwork being in order at every mammography site in the U.S., but fail to ensure that various FDA-approved digital mammography acquisition and display workstations are compatible in terms of image display."
By Kate Madden Yee
AuntMinnie.com staff writer
February 2, 2010
Related Reading
Breast MRI best for finding mammographically occult cancers, November 9, 2009
Chest MDCT offers value as breast imaging tool, September 2, 2009
Breast imagers, ahoy: New technology on the horizon, August 20, 2009
CARS report: Novel conebeam CT cuts breast dose, improves accuracy, June 29, 2009
Ultrasound-guided marker placement improves breast lesion identification, December 30, 2008
Copyright © 2010 AuntMinnie.com




















