Breast implants filled with silicone gel have a "reasonable assurance" of safety and effectiveness when used as labeled, but women still should have routine follow-ups, including breast MRI scans, according to a report issued on Wednesday by the U.S. Food and Drug Administration (FDA).
The agency's updated report is based on preliminary data from postapproval studies conducted by breast implant manufacturers Allergan and Mentor, as well as reported adverse events and recent clinical publications. The FDA approved silicone gel-filled breast implants in November 2006 for breast augmentation in women older than 22 years and for breast reconstruction in all women.
The report emphasized that women should understand the risks prior to considering silicone breast implants for breast augmentation or reconstruction. In addition, women should realize that these implants are not lifetime devices, said Dr. Jeffrey Shuren, director of the FDA's Center for Devices and Radiological Health (CDRH), during a conference call about the report.
As many as one in five women who receive silicone gel-filled breast implants to increase the size of their breasts through augmentation will need to have the implants removed within 10 years. As many as one in two women who receive the implants to replace breast tissue that was removed due to disease or trauma reconstruction will need to have those implants removed within the same time frame, Shuren added.
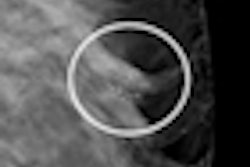
The studies found that silicone gel-filled breast implants are associated with significant local complications and outcomes, including capsular contracture (hardening of the area around the implant), additional surgeries, or implant removal. Other frequent complications include implant rupture, wrinkling, asymmetry, scarring, pain, and infection. The new data indicate that the longer a woman has a silicone implant, the more likely she is to experience local complications.
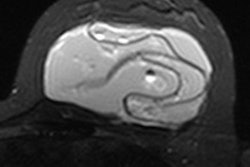
Women should have routine checkups, including breast MRI scans, to detect silent rupture of the breast implant.
The new data did not identify any new risks related to implants, with the exception of a possible small risk for a rare type of cancer called anaplastic large cell lymphoma, which the FDA announced in January.
"To date, preliminary data does not indicate that silicone gel-filled breast implants cause breast cancer, reproductive problems, or connective tissue disease, such as rheumatoid arthritis or lupus," Shuren said. "However, in order to rule out these and other rare complications, studies will need to be larger and longer than those conducted thus far."
The FDA report also recommends that women notify their healthcare professionals if they develop any unusual symptoms, and all serious side effects should be reported to the breast implant manufacturer and MedWatch, the FDA's safety information and adverse event reporting program.