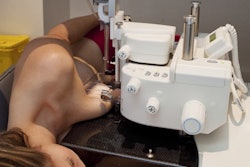
Breast cancer treatment technology provider Devicor Medical Products has received U.S. Food and Drug Administration (FDA) clearance for Mammotome revolve, a new dual vacuum-assisted biopsy system.
Mammotome revolve features an advanced specimen management system that can collect and organize high-quality individual tissue samples in numbered chambers, designed to preserve their integrity for specimen radiography and pathology studies. It also features Devicor's DualVac vacuum technology that enables clinicians to secure larger contiguous tissue samples.
Mammotome revolve also includes a physician-controlled variable aperture that enables users to confirm or adjust sample acquisition response, a benefit for accessing and acquiring challenging lesions, as well as for reducing prebiopsy planning time.
The unit is currently in limited market release in the U.S., Canada, and Europe and is expected to be in full release in the spring of 2013.