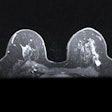
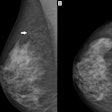
Siemens Healthineers has received U.S. Food and Drug Administration (FDA) approval for the use of 3D-only screening mammography on the company's Mammomat Inspiration digital mammography system with a tomosynthesis option.
Siemens' tomosynthesis-only option is available on the company's Mammomat Inspiration and Mammomat Inspiration Prime Edition digital mammography systems.
FDA approval of the 3D-only screening application follows a reader study in which participating radiologists demonstrated their ability to increase cancer detection at a lower radiation dose than with combined 2D and digital breast tomosynthesis. Radiologists also decreased average recall rates by an average of 19% without the need for a 2D image, Siemens said.