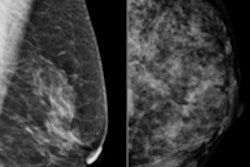
In its ongoing efforts to improve breast cancer detection rates, the U.S. Food and Drug Administration (FDA) has announced that it plans to use its Mammography Quality Standards Act (MQSA) inspection program to increase mammography image quality through a new venture it's calling Enhancing Quality Using the Inspection Program (EQUIP).
Dr. Helen Barr, director of the agency's Division of Mammography Quality Standards, described the program at an MQSA meeting in September. It will begin in January 2017 with educational inspections that will give sites the opportunity to verify whether their program meets EQUIP's requirements. No citations will be given in this first year.
Why now?
What is the motivation behind EQUIP? Clinical images are the primary reason for accreditation failures, with positioning as the most common issue, followed by lack of appropriate quality control processes when imaging parameters are not adequate.
 Bonnie Rush from Breast Imaging Specialists.
Bonnie Rush from Breast Imaging Specialists.However, since accreditation only occurs every three years, tracking image quality in the interim can be a challenge. I've seen this many times as a consultant: A site is about to go through reaccreditation and realizes it needs a positioning update; or, more commonly, it has failed its accreditation and now needs to provide remedial positioning training. To avoid these scenarios, facilities should evaluate mammography image quality daily -- and, if needed, bring in a trainer on a regular basis.
The EQUIP initiative will use three questions to evaluate a facility's image quality and corrective action protocols:
- Does the facility have a quality assurance program for clinical image review, and a plan for corrective action when images do not meet requirements? The inspector will make sure that there is a system in place, and although a facility does not have to have written policies and procedures, any individual involved with clinical image review has to be able to verbally explain the system to the inspector. This part of the EQUIP program stresses that the ultimate responsibility to determine viability of images lies with each involved interpreting physician, with mandated ongoing feedback on image quality. Although it does not suggest a mechanism to provide feedback and/or corrective action, it does require written documentation of the reason for any corrective action and whether it was effective.
- Does the facility's clinical image quality procedure comply with standards from an accrediting body for clinical image review, which includes positioning, compression, exposure level, contrast, sharpness, noise, artifacts, and examination identification? Once again, the FDA will leave it to the facility to establish this procedure, but it must be documented and include a mechanism for regular review of a random sample of images by all involved technologists, as well as a sample of mammograms that were accepted for interpretation by each involved interpreting physician. This process must be documented as reviewed at least annually, although the program encourages that it be done more regularly. Facilities can show compliance through a summary report with a signed statement by the lead interpreting physician, clinical image review meeting records, and memos of review results to radiologic technologists and interpreting physicians.
- Does the facility's quality control process require that the lead interpreting physician oversee quality assurance/quality control records and any needed corrective actions? This oversight includes setting review frequency. Although it is not required to be a written program, it is required that corrective action be documented when performed.
Take action
Sites should address the adequacy of their program now, and if there is a need for corrective action to update imaging quality, get outside help. Let's acknowledge -- before there is a failure, either through an inspection citation or through a false-negative interpretation -- that high image quality is essential to our screening programs.
Bonnie Rush is president of Breast Imaging Specialists of San Diego and author of MQSA Made Easy, Understanding and Implementing the Facility-Based Final Regulations.
The comments and observations expressed herein are those of the author and do not necessarily reflect the opinions of AuntMinnie.com.