CHICAGO - In its booth at RSNA 2017, GE Healthcare is highlighting a wide range of product launches and upgrades, from a new CT scanner designed to make it easier to perform routine spectral imaging to new PET/CT and SPECT/CT systems.
In between, the vendor is showing a mammography system with patient-controlled compression, presenting new lightweight MRI coils, and discussing ongoing work on a 7-tesla MRI system.
CT
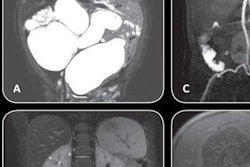
In the CT section of its booth, GE is launching Revolution Frontier, a new 128-slice scanner with a 0.35-sec gantry rotation speed designed to make it even easier to perform spectral CT imaging. Frontier features a new imaging chain, Gemstone Clarity detector, and Performix HD Plus x-ray tube; the combination of these technologies enables the scanner to achieve anatomic detail of 0.23 mm with 25% less electronic noise, according to the company.
 Revolution Frontier features improvements in spectral imaging.
Revolution Frontier features improvements in spectral imaging.For spectral CT, Frontier is outfitted with the next generation of the company's Gemstone spectral imaging package, GSI Pro. The technology sports a reconstruction speed that's twice as fast as the previous GSI software for routine spectral workflow, while the company's ASiR-V adaptive statistical iterative reconstruction algorithm is available to reduce dose.
Revolution Frontier has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) but is awaiting the CE Mark. The company expects shipments to begin in the second quarter of 2018.
GE is also launching a new program called Smart Subscription that's designed to make it easier for users to gain access to the company's latest upgrades and enhancements through a single annual fee for each device.
GE believes that Smart Subscription will help its customers avoid having equipment become obsolete over the life cycle of a system by keeping it upgraded with the latest software. It will also help ensure that a healthcare enterprise's CT fleet has the same capabilities at all sites, while improving staff efficiency, reducing training requirements, and improving customer satisfaction by ensuring there is one set of capabilities to learn, operate, and read.
Two applications will be available under Smart Subscription when it launches in the first half of 2018 -- Smart MAR, GE's metal artifact reduction protocol, and the ASiR-V iterative reconstruction package. The program is first being launched on the Optima CT 660 scanner, and it will be rolled out to other systems in GE's CT portfolio.
MRI
In MRI, GE is highlighting Signa Premier, a 3-tesla MRI scanner that received 510(k) clearance in August. The scanner is the product of a collaboration between GE, the NFL, and global research institutions to use MRI to help find biomarkers to detect signs of traumatic brain injury.
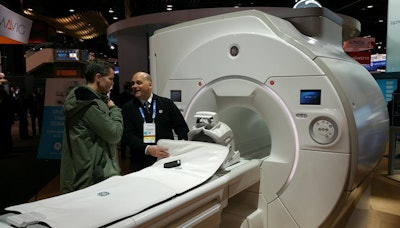 Signa Premier was developed in part with a grant from the NFL.
Signa Premier was developed in part with a grant from the NFL.GE and the NFL announced the collaboration in 2013 as part of a $60 million research deal to develop imaging technologies to better detect and diagnose concussions, with $40 million set aside for imaging modalities and $20 million devoted to better helmet design.
Signa Premier features a 70-cm short-bore superconducting magnet, with the company's SuperG gradient coil, which is designed to provide the performance of a research-class 60-cm MR system in a scanner with a 70-cm bore. The system also has GE's digital radiofrequency (RF) transmit and receive architecture, which provides 146 independent receiver channels that simultaneously acquire patient data from multiple high-channel-density surface coils, enabling faster scanning and better image quality. In other technical specifications, the scanner's gradients are rated at an amplitude of 80 mtesla/m and a slew rate of 200 tesla/m/sec.
Premier also supports GE's AIR Technology for RF coils, another show highlight for the company. AIR coils are lightweight and flexible, allowing them to be positioned in ways not possible with older coil technology. AIR elements function independently and are electrically immune to the surrounding environment or other neighboring elements. Component volume is reduced by more than 60%, and there is 95% more transparency for attenuation correction than with conventional MRI coils. The coils have received 510(k) clearance.
 GE's AIR RF coils are designed to be lightweight and flexible.
GE's AIR RF coils are designed to be lightweight and flexible.Finally, while GE didn't show a gantry on the RSNA show floor, the company is discussing its development of a 7-tesla MRI scanner. The system will be based on the Premier platform and will share that system's 146 digital RF architecture and 70-cm magnet bore. GE hopes to secure 510(k) clearance for the system so it can be marketed for clinical use.
Interventional x-ray
In interventional radiology, GE is showing its Discovery IGS 7, a wide-bore conebeam CT (CBCT) system designed to make 3D imaging easier to perform regardless of patient size, arm position, or intubation. The company is also touting its Liver Assist and Needle Assist software: Liver Assist helps clinicians perform embolization for surgical resection as well as palliative treatments such as endovascular embolization of vessels that feed the tumor. Needle Assist offers real-time visualization of needle positions during interventional procedures.
Also on display in GE's booth is new software that helps reduce involuntary respiratory motion artifacts in interventional radiology. Motion Freeze reconstructs CBCT acquisitions affected by patient breathing and is incorporated into GE's AW Workstation.
The company is also highlighting a series of software packages that allow interventional radiologists to access any preoperative images taken across a variety of modalities: Interact CT, Interact MR, Interact General Imaging Ultrasound, and Interact Cardiovascular Ultrasound.
Finally, GE is touting a hybrid operating room (OR) that was developed with Getinge. The room combines the Discovery IGS 7 OR mobile robotic gantry with Getinge's Maquet Magnus OR table system.
Ultrasound
On the ultrasound side, GE is showcasing a number of improvements to its existing line of devices. For example, the company's Logiq S8 unit now includes XDclear 2.0, a new version of GE's image processing engine, and French ultrasound technology developer Echosens' FibroScan technology, which helps clinicians diagnose various types of liver disease, assess fibrosis stage in chronic liver disease, and monitor treatment.
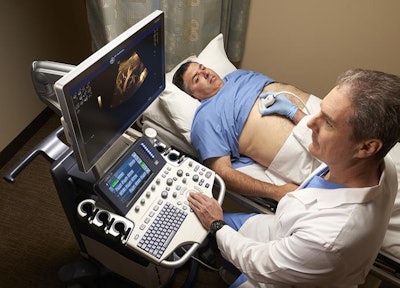 Logiq S8 now includes XDclear 2.0 and FibroScan. Image courtesy of GE.
Logiq S8 now includes XDclear 2.0 and FibroScan. Image courtesy of GE.GE's Logiq E9 XDclear 2.0 now includes its Agile Acoustic platform and display technologies, which improve its spatial resolution by 99% and increase image information by 170%, compared with previous versions of the device, the company said. Its Logiq P9 system now has an offline battery life of one hour, making it easy to use throughout the hospital. And GE's Voluson E10 system has been upgraded to include a number of new technologies, including an electronic 4D probe designed for ob/gyn applications; Radiantflow, a color visualization tool; and the next generation of HDlive, its sonographic imaging rendering technique.
Finally, GE is highlighting Vscan Extend, a handheld ultrasound device with Wi-Fi and DICOM capabilities. Vscan Extend is linked to GE's cloud, where the company hosts apps through its Marketplace that allow physicians to access measurement and protocol tools.
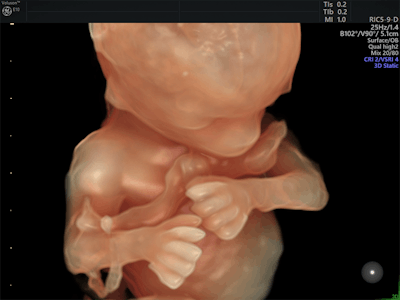 Fetus at 14 weeks with Voluson E10 and HDlive. Image courtesy of GE.
Fetus at 14 weeks with Voluson E10 and HDlive. Image courtesy of GE.Imaging informatics
In the imaging informatics section of its booth, GE is discussing a collaboration with the University of Pittsburgh Medical Center on an algorithm called Imaging Related Clinical Context (IRCC), which is designed to deliver relevant patient context to radiologists when they are reviewing images. This includes surgical notes, pathology reports, and clinical notes, which are delivered directly to radiologists and embedded in their existing workflow. The IRCC algorithm learns from radiologists via keywords and sentence structure how to select relevant clinical data. It is expected to be commercially available in 2018.
GE is also showing machine-learning algorithms it has developed with a number of academic institutions, including an algorithm designed to detect pneumothorax that teaches machines to distinguish between normal and abnormal imaging studies and prioritize cases in which the patient may be in critical condition. Another algorithm is designed to detect signs of stroke and help radiologists prioritize abnormal findings. The applications should be available in the fourth quarter of 2018.
Another option being developed is Native Breast Imaging in Centricity Universal Viewer, which is designed to provide native breast screening and diagnostic workflows in a single viewer that can be used for all modalities. Meanwhile, GE's CT myocardial perfusion application has been added to the company's suite of cardiac applications, while another new addition is ProView for guided reporting of multiparametric MRI prostate exams.
The company is also showing enhancements to its existing portfolio of software, including its Centricity Imaging Collaboration Suite, AW VolumeShare 7, and Centricity Cardio Enterprise.
Women's imaging
For women's imaging, GE is emphasizing its efforts to increase breast cancer screening compliance by making mammography exams more comfortable and effective. This effort includes its Senographe Pristina system, launched at last year's RSNA meeting, but now with Pristina Dueta, a handheld wireless remote control that allows women to adjust the amount of compression during the exam after the technologist has established baseline, clinically effective compression. Dueta was cleared by the FDA in September.
Senographe Pristina uses the company's Seno Iris workstation, and it can be combined with GE's SensorySuite, a room designed to reduce patient anxiety via nature images and sounds. It is also available as a mobile unit, the company said.
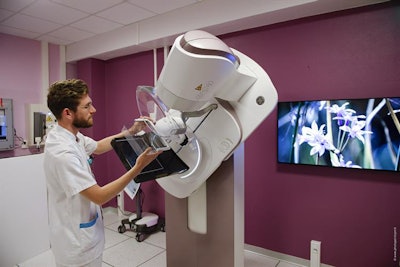 GE's Senographe Pristina in the SensorySuite. Image courtesy of GE.
GE's Senographe Pristina in the SensorySuite. Image courtesy of GE.Also in GE's booth is SenoBright HD, a contrast-enhanced spectral mammography capability available on Senographe Pristina that offers an alternative to breast MRI following an inconclusive mammogram or ultrasound. Patients receive an iodine contrast agent and undergo a standard four-view mammogram; the contrast agent highlights areas of unusual blood flow. The exam takes less than seven minutes, and images are immediately available for radiologist review, GE said.
Finally, the company is highlighting its Invenia automated breast ultrasound (ABUS) unit, which features a curved transducer to accommodate the breast. It is also introducing Senographe Crystal Nova, an affordable digital mammography unit that uses the same carbon fiber image detector as Pristina.
X-ray
For radiography, GE is introducing Helix, an advanced image processing package that complements its FlashPad HD digital detectors. The combination of Helix with FlashPad HD improves contrast detection by up to 40%, according to the firm. The company is working on developing artificial intelligence algorithms for Helix that will help technologists obtain the most critical images, as well as an app that will help radiologists prioritize which studies to read by flagging those that need to be interpreted quickly.
GE has incorporated Helix into its newest premium fixed digital radiography (DR) system, Discovery XR656 HD, as well as its most recent mobile x-ray device, Optima XR240amx with FlashPad HD. Both Discovery XR656HD and Optima XR240amx are compatible with GE's new x-ray quality application, which features what the company calls Repeat/Reject Analytics (RRA). RRA automatically mines data from a facility's x-ray devices to help users get the best possible image the first time and gather information about why images are rejected.
The company is also showcasing its work-in-progress Discover RF 180, a remote imaging system for radiology and fluoroscopy that features a 705-lb table capacity. Clinicians can perform tomosynthesis, stitching, digital subtraction angiography, bariatric exams, and radiographic exams without additional equipment, GE said.
Finally, GE is touting its Proteus XR/c, a ceiling-mounted compact digital x-ray system that includes wireless Konica Minolta AeroOR detectors and can support up to 770 lb. Proteus XR/c is not yet commercially available in the U.S.
Molecular imaging
In molecular imaging, GE is highlighting the Discovery MI PET/CT scanner that was introduced in 2016 -- 100 orders and 40 systems have been installed globally since then. The system features GE's time-of-flight and Q.Clear reconstruction technologies. At RSNA 2017, GE is introducing a three-ring 15-cm axial field-of-view option for the system, which can be upgraded to a four-ring 20-cm field-of-view after installation to give hospitals additional flexibility in purchasing options.
GE is also introducing Discovery MI DR, a digital-ready PET/CT scanner that supports cardiac and brain imaging and can be upgraded.
In SPECT, the company is highlighting its Discovery NM/CT 670 CZT SPECT/CT system, based on cadmium zinc telluride (CZT) digital detectors, and Discovery 670 DR, a SPECT/CT unit introduced in 2017 that can be upgraded to CZT technology within a facility. Both systems feature 16-slice CT scanners that use overlap reconstruction technology to achieve 32 slices per rotation.