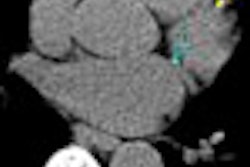
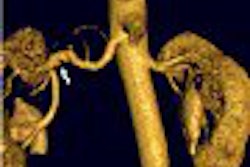
Cardiac technology developer Biosense Webster of Diamond Bar, CA, and Johnson & Johnson subsidiary Biologics Delivery Systems Group, Cordis has received U.S. Food and Drug Administration clearance to market a cardiac 3D imaging device, the Noga XP cardiac navigation system.
The Noga XP is built on real-time electroanatomical mapping data received from a magnetic reference system that integrates software and catheter, the companies said.
Biosense Webster developed and manufactured the Noga XP cardiac navigation system. Biologics Delivery Systems Group, Cordis of Miami Lakes, FL, will distribute the technology, according to the companies.
By AuntMinnie.com staff writers
March 16, 2006
Related Reading
Cordis launches Palmaz Blue stent in U.S., July 1, 2005
Cordis debuts Tempo Aqua catheter, June 14, 2005
FDA approves Cypher stent with MRI scans, April 21, 2005
Medtronic loses verdict in Cordis trial, March 15, 2005
Court denies Cordis preliminary injunction, May 31, 2004
Copyright © 2006 AuntMinnie.com