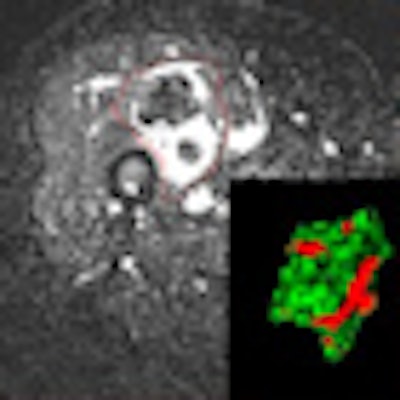
Software developed at Massachusetts General Hospital (MGH) may offer a better way to estimate changes in tumor volume after treatment in soft-tissue sarcoma patients, giving physicians a better tool to track the effectiveness of therapy.
As a result, the MGH-developed software shows potential to make up for shortcomings in the Response Evaluation Criteria in Solid Tumors (RECIST) and the need for pathology tumor viability estimates to be performed using all cut sections of an excised tumor. The research was led by Dr. Anand Singh, who presented the results at the American Roentgen Ray Society (ARRS) annual meeting earlier this year.
"This could provide an automated and more objective way to assess [the remaining] viable portions in the tumor, so that the medical and radiation oncologists could plan further treatment workups after tumor resection," he said.
RECIST limitations
In a clinical setting, medical and radiation oncologists rely on surgical pathology estimates when deciding about remaining treatment cycles for excised tumors. Therefore, if appropriately validated, an automated, imaging-based quantification method may eliminate the problems associated with RECIST and the limitation of generating tumor viability estimates on all cut sections on pathology, Singh said.
Versions 1.0 and 1.1 of the RECIST criteria for assessing oncology therapy response are based on maximum 1D tumor measurements from calipers on images. As such, they are subject to intraobserver and interobserver variability, and users of the criteria routinely encounter limitations, Singh said.
For example, measurements based on RECIST may lead to false-positive interpretation of disease progression in tumors where the increase in tumor size is secondary to an increase in necrotic fluid within the tumors after treatment. This dynamic is one of the changes experienced after a round of therapy, he said.
"Moreover, in some recent studies that evaluated targeted drugs or radiation treatments, little or no correlation between tumor response and survival has been shown, and thus current evaluation criteria based on morphology of tumors have limited ability for evaluation of these targeted therapies," Singh told AuntMinnie.com. "Thus, the practical use of RECIST, together with the rapid development of imaging techniques and newer chemotherapeutic agents, has highlighted its limitations and the need for an updated criterion."
In a proof-of-concept, retrospective study in 2010 supported by a grant from the Sarcoma Foundation of America, the researchers applied image postprocessing techniques on preoperative MRI datasets of patients who were treated for soft-tissue sarcomas by radiation and/or chemotherapy, Singh said. They then studied the correlation between 3D MRI-derived viable tumor estimates on these preoperative MRI datasets and pathology estimates for tumor viability reported on the excised tumor specimens.
Prospective trial
Results from the retrospective project were encouraging enough to warrant a prospective study, which began in 2011 in a collaboration between MGH's department of imaging and the MGH Center for Sarcoma and Connective Tissue Oncology (CSCTO).
Singh and colleague Wenli Cai, PhD, decided to test their 3D technique to estimate viable portions of tumors in post-treatment MRI scans of sarcoma patients who had undergone surgery.
Preliminary data had shown that nondynamic images of soft-tissue sarcomas had led to enhancement of dead tumor components that could be picked up by the 3D software during generation of viable tumor volumes. So the researchers performed a prospective study using a new acquisition approach: Dynamic (functional) postcontrast MRI images were acquired on patients who completed treatment for sarcoma with radiation or chemotherapy after resection. The study received funding from the National Institutes of Health (NIH) MGH Proton Beam Federal Share program.
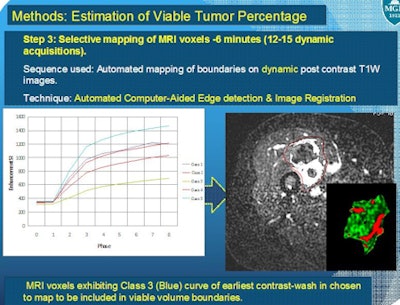
In 10 patients who underwent treatment for soft-tissue sarcoma, dynamic postcontrast 15- to 17-sec acquisitions were consecutively obtained for seven minutes after the start of contrast injection. This allows the software to provide automated generation of tumor contrast wash-in and wash-out curves.
The enhanced areas that matched the curves for early-contrast wash in and wash out were included in the estimation of viable tumor burden by the MGH-developed computer-aided functional MRI workstation.
 |
The same 10 patients also underwent a delayed contrast acquisition, which was performed after the seven-minute acquisition of the dynamic series had ended. This allowed comparison of both the nondynamic and dynamic contrast acquisitions with the pathology estimates.
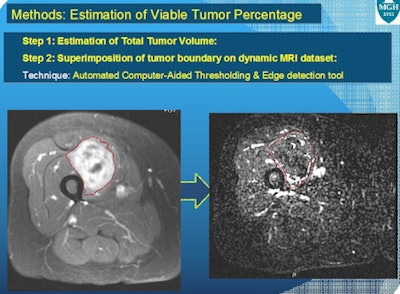
Total tumor outline was first determined on a T2-weighted image and registered on dynamic postcontrast acquisitions. Thanks to in-house 3D software programming, only the contrast-enhanced MRI voxels that corresponded to the early wash in and wash out of contrast were displayed. Their volume was then evaluated.
 |
The researchers also obtained the volume of contrast-enhancing tumor fraction from nondynamic postcontrast acquisitions in the same patients. Pathology sections on these specimens were performed axially, and the levels of sections from the proximal and distal ends of tumors were noted, according to Singh and colleagues.
The team then compared the MRI viable tumor fraction estimates from the dynamic and nondynamic contrast acquisitions with pathology estimates derived from the same level.
Correlation analysis revealed that estimates from dynamic MRI had a strong correlation (r = 0.87, p = 0.0012) with pathology tumor fraction estimates, while estimates from nondynamic MRI did not (r = 0.4218, p = 0.2247).
"Dynamic MRI-pathology correlation of viable tumor was much better than nondynamic MRI-pathology correlation for viable tumor," Singh said.
Future steps
The researchers now plan to prospectively evaluate the software in a set of 40 patients to generate more clinical data, in hopes of making the technique available for use in clinical trials for assessing therapy response in sarcomas.
"We also plan to generate a peripheral tumor burden to facilitate resection of these tumors where surgeons will be able to identify the tumor edge with increased viable tumor load and accordingly plan a wider excision on that side of lesion, so that viable tumors cells are not left in patients after excision," he said. "We thus envision a decrease in recurrence rates of these tumors."
The software technique is not specific to musculoskeletal tumors and could potentially be used for objective assessment of treatment response for tumors in other parts of the body, Singh also noted.