Advanced visualization firm Vital Images has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its fenestrated stent planning tool.
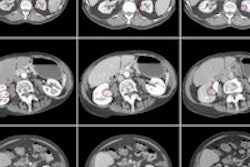
The addition of fenestrated stent planning workflow to the company's endovascular stent planning (EVSP) application supports patients who don't meet the recommended criteria for endovascular aortic aneurysm repair due to inadequate infrarenal neck anatomy, Vital Images said. With fenestrated stent planning, surgeons can make precise measurements for determining the size and placement of fenestrated stents tailored to each patient's specific anatomy, according to the vendor.
A clock-angle tool provides measurements, including arc lengths, and surgeons can also customize their workflows using stent template editing tools, the company said. CT EVSP with fenestrated stent planning is available worldwide in version 6.7 of Vital Images' Vitrea software.