Advanced visualization developer EchoPixel is moving its True 3D Viewer technology to market after receiving clearance from the U.S. Food and Drug Administration (FDA) for the platform, which creates holographic displays from CT and MRI data.
True 3D is designed to address a problem inherent in medical imaging: Cross-sectional scans are fundamentally 2D representations of 3D objects. Radiologists have learned to translate 2D slices into 3D volumes in their minds, but this simply creates extra work for radiologists, according to Sergio Aguirre, chief technology officer of the firm.
"We want them not to have to solve a 3D problem and then solve the clinical problem; we want them only to solve the clinical problem from the start," Aguirre said.
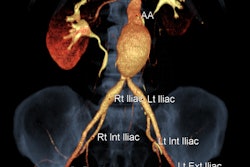
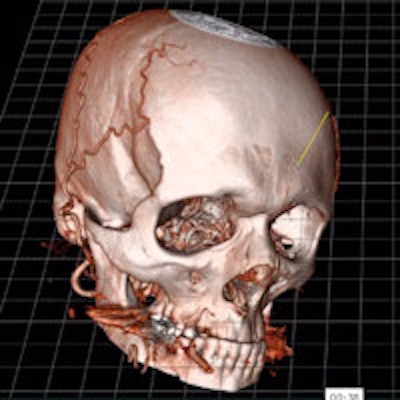
True 3D takes data from CT and MRI scanners, processes it with EchoPixel's proprietary algorithms, and then outputs it to an off-the-shelf 3D display to create holographic volumes that appear to float in space. The volumes are fully interactive, and users can manipulate and interact with images, turning them around in space, performing measurements, and "touching" volumes with a special stylus.
True 3D is being used at a number of clinical sites, including Stanford University and the University of California, San Francisco (UCSF). At UCSF, it is being used by Dr. Judy Yee to develop navigation techniques for traversing the colon and identifying polyps. Yee has found that True 3D is well-suited for identifying polyps and in particular flat lesions, and a clinical trial for virtual colonoscopy is in the works.
At Stanford, Dr. Frandics Chan has used EchoPixel's technology in preliminary clinical trials for surgical planning. In a study presented at RSNA 2013, he found that using the platform helped improve surgery for children with pulmonary atresia with major aortopulmonary collateral arteries by increasing detection sensitivity from 81% to 90% and reducing interpretation times from 22 minutes to 13 minutes, a 40% improvement.
Indeed, EchoPixel sees surgical planning as one of the major clinical applications for True 3D. In addition to helping plan the particulars of a surgical intervention, True 3D can open up a channel of communication between surgeons and radiologists -- and even patients -- by representing patient anatomy in a form that's easier for everyone to understand.
EchoPixel was incorporated in 2012, and the firm has displayed True 3D at several trade shows since then, including RSNA meetings and the annual Society for Imaging Informatics in Medicine (SIIM) show. The firm's chairman and CEO is medical imaging veteran Ron Schilling, PhD, who has also been involved in other pioneering medical imaging firms such as Diasonics, Toshiba America Medical Systems, and GE Healthcare.
The company has planned a three-pronged commercialization strategy for the U.S., with one-third of sales handled directly through its own sales executives, one-third through distributors, and one-third through corporate partners. A perpetual license to True 3D costs $75,000, but the company also has a subscription model that costs about $20,000 annually; the firm is also working on a pay-per-study approach, as well as a cloud-based model.
International sales began about a year ago; the company recently secured a very large order from Saudi Arabia, and sales are also underway in Australia and New Zealand. The health division of Chinese contract manufacturer Foxconn -- which make iPhones for Apple -- is also trying out several systems, which could lead to an order, Schilling said.
Ultimately, Schilling sees patient demand driving sales of the system, due to its ability to make abstract anatomical concepts clear in a way that's not possible with traditional cross-sectional imaging.
"How do you explain to a patient who is having a shoulder problem whether they should have surgery, and you are showing them 2D images?" he said. "It's a difference of night and day."