Swedish cranial and facial reconstruction implant firm OssDsign said it has received U.S. Food and Drug Administration (FDA) clearance for its flagship OssDsign Cranial product for reconstructing defects during cranioplasty via 3D-printed implants.
The clearance means the company can continue working on its existing plan to launch the product in the first quarter of 2017, OssDsign said. It is in the final stages of setting up a distribution network that will enable it to bring the product's benefits to surgeons, patients, and healthcare systems in the U.S.
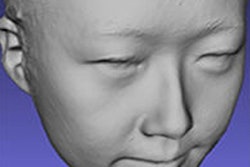
OssDsign Cranial is a patient-specific medical implant for reconstructing cranial defects. Cranioplasty is traditionally performed by neurosurgeons using the patient's own bone or by using implants made from inert plastic or metal materials such as titanium. However, these techniques have high rates of complications, especially infections, which can sometimes lead to removal of the implant and additional surgery, the company said.
OssDsign Cranial implants are designed from CT data using the company's 3D printing technologies to create a unique device for each patient from a proprietary calcium phosphate composite, reinforced with a titanium skeleton for strength.
Introduced in Sweden two years ago, the product has good handling characteristics leading to positive outcomes in complex cases, the company said. OssDsign products are already available in Germany, the U.K., and Nordic countries, as well as in Singapore and Israel. Earlier this month, the company also announced new commercial partnerships to distribute its medical implants in Italy, Spain, Switzerland, Austria, and the Netherlands.