A multinational research group has used 3D reconstruction of micro-CT images to visualize -- for the first time in 3D -- the group of cells that cause the heart to beat. The method shows promise for helping cardiologists plan heart surgeries and achieve better patient outcomes.
Using volume rendering and segmentation techniques on contrast-enhanced micro-CT images, researchers led by Dr. Robert Stephenson of Aarhus University in Aarhus, Denmark, and Andrew Atkinson of the University of Manchester in Manchester, U.K., have created 3D representations of the cardiac conduction system on an intact human heart. What's more, the group incorporated the anatomical data into mathematical simulations that may yield insights into the manifestations of arrhythmias and cardiac conduction disturbances.
"This technique promises to have considerable impact on the understanding, and strategies, associated with ablation and reconstructive surgery in diseased and congenitally malformed hearts," the authors wrote in their paper, which was published online August 3 in Scientific Reports. "It should reduce the incidence of conduction abnormalities postintervention and, thus, reduce patient morbidity and the need for expensive pacemaker implantation."
Other members of the team were from Liverpool John Moores University in Liverpool, U.K.; Newcastle University in Newcastle, U.K.; the National Institute of Legal Medicine in Bucharest, Romania; the Visible Heart Laboratory at the University of Minnesota in the U.S.; and the University of Auckland in Auckland, New Zealand.
Cardiac conduction system
Functioning as a kind of biological pacemaker, the human cardiac conduction system maintains a heart rate of 60 to 100 beats per minute. The system, a group of cardiac muscle cells in the wall of the heart that can't be visualized using conventional noninvasive techniques, includes several main components. The sinus node of the right atrium serves as the primary pacemaker, while the atrioventricular conduction axis forms the only pathway that allows excitation to pass between the atria and the ventricles, according to the researchers. The last main component, the Purkinje network, enables coordinated conduction within the ventricles.
If the activity to the conduction system is disrupted by disease or injuries to the heart, a marked increase or decrease in heart pumping function may result -- often accompanied by an irregular pumping pattern, according to a statement from Aarhus University. These abnormal conditions can also lead to a higher risk of systemic clots, and patients can develop arrhythmias such as atrial fibrillation.
Knowledge of the 3D microanatomy of the cardiac conduction system becomes very important when invasive surgeries are performed to treat these cases, but this information has been absent from the literature, according to the authors.
Clearing up controversies
The literature and medical textbooks provide mixed -- and often inaccurate -- descriptions of both the cardiac conduction system and the arrangement of these specialized cardiomyocytes, or cardiac muscle cells, according to Stephenson and colleagues.
"In order to provide definitive answers to these existing controversies, there is the need to present the microstructure of the whole heart in three dimensions at cellular resolution," the authors wrote. The nondestructive imaging method of micro-CT can provide such datasets in less than an hour of scanning time.
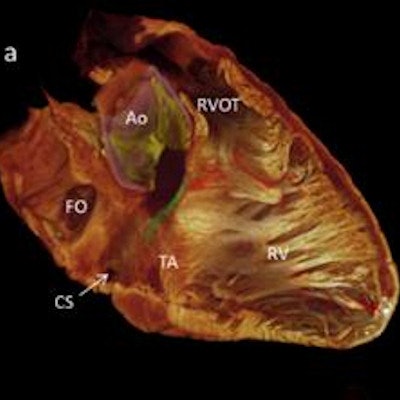
Following up on previous work that showed the 3D distribution of the cardiac conduction system in animal hearts, the researchers performed contrast-enhanced micro-CT on two human hearts that had been obtained postmortem from organ donors. Although they were considered to be anatomically normal, the hearts had been deemed unsuitable for transplantation. After open-source ImageJ image processing software was used to view, crop, and manipulate the image datasets, 3D reconstructions of regions of interest were performed with version 5.33 of the Amira software (FEI Visualization Sciences Group). Volume rendering and segmentation techniques were combined, enabling segmentations of the major regions of the cardiac conduction system to be overlaid on the volume renderings.
"Although histologically validated, the 3D digital reconstructions represent our hypothesis of the relevant anatomical features of the cardiac conduction system," the authors wrote.
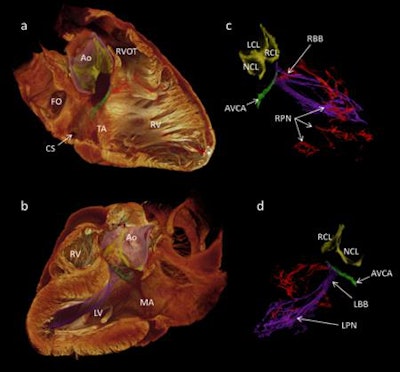 3D visualization of the cardiac conduction system of a healthy human heart, generated via 3D reconstruction of micro-CT image data. Image courtesy of Aarhus University.
3D visualization of the cardiac conduction system of a healthy human heart, generated via 3D reconstruction of micro-CT image data. Image courtesy of Aarhus University.The group then used the segmentation dataset to develop a mathematical model of electrical activation in the heart.
A practical method
The researchers believe their method will have a multidisciplinary impact. As cardiac microstructure and the precise orientation of the cardiac conduction system dictate the rates of depolarization, the technique should improve the fidelity of mathematical models of cardiac conduction, according to the group.
The approach also shows potential for improving patient care. For example, the ability to show precise 3D relationships between the cardiac conduction system and surrounding structures provides new insights relevant to valve replacement surgery and ablation therapies.
"We also offer a practical method for investigation of remodeling in disease and, thus, virtual pathology and archiving," they wrote. "Such data presented as 3D images or 3D-printed models will inform discussions between medical teams and their patients, and aid the education of medical and surgical trainees."
In addition to enhancing understanding and treatment of diseased and congenitally malformed hearts, the technique shows potential for lowering patient morbidity by reducing the incidence of conduction abnormalities following ablation and reconstructive surgery. As a result, these patients would be less likely to require implanted pacemakers, according to the authors.
In the next phase of their work, Stephenson and colleagues plan to generate 3D visualizations of diseased hearts.