The COVID-19 pandemic has led to a severe shortage of testing equipment and personal protective equipment (PPE) for healthcare workers. 3D printing labs in radiology departments can help meet this challenge, according to a webinar held by the RSNA's 3D Printing Special Interest Group on April 24.
In talks during the webinar, leaders of three radiology 3D printing labs shared their experience with producing 3D-printed face shield holders, surgical face masks, and nasopharyngeal swabs.
Face shields
The 3D Imaging Lab in the department of radiology at Montefiore Medical Center
City in New York City has converted completely to printing face shield holders during the COVID-19 crisis, according to Nicole Wake, PhD, director of 3D imaging.
Wake was inspired after seeing media reports on how Italian engineers had 3D-printed Venturi valves for respirators.
"Being in the 3D Imaging Lab with access to 3D printing, I started thinking [about] what I could do to help," she said.
 Nicole Wake, PhD, director of 3D imaging at Montefiore Medical Center.
Nicole Wake, PhD, director of 3D imaging at Montefiore Medical Center.Initially, she worked with 3D printing firm 3D Systems to create her own Venturi valves, and she also started printing hands-free door openers from 3D printing software developer Materialise. In addition, Wake printed door openers at her home using an Original Prusa i3 MK3 printer from a design by Sarah Flora, director of 3D imaging at Geisinger Healthcare. She also has two other 3D printers at home that were loaned to her by Ultimaker.
Wake experimented with several mask prototypes, but she quickly decided that would be a difficult project. After hearing on March 22 about a couple in upstate New York who had been 3D-printing face shields, Wake decided to focus on that vital piece of equipment using 3D-printing designs from Budmen and Prusa.
The face shield designs have two parts that need to be assembled after printing. As a result, the 3D lab was essentially transformed into a factory of sorts, Wake said. Radiology residents began rotating through the lab and helped to assemble the shields.
"We started out printing the Prusa model, since to us that seemed more comfortable," she said.
New designs
However, they switched over to the Budmen design after receiving feedback from their hospital's environmental team that splatter could too easily get through the Prusa design. Wake said she also printed a lot of face-shield models using a design from Ultimaker.
"The advantage of this design is that we could print six in one build plate in about two hours," she said. "So it was fairly fast."
The institution has also received many donations of 3D-printed shields, including from 3D Systems, Stratasys, and Carbon.
"We were even assembling shields at home," she said.
Wake said that she's currently using the shield design from 3DVerkstan on its Ultimaker printers. Face shield production has become a constant effort for the lab.
"People found out that we were making these and would come to the lab and ask for shields when they found out they were going to see a patient with [COVID-19]," she said. "We started delivering shields to many departments, such as medicine, critical care, emergency medicine. We delivered boxes to the emergency departments, to CCUs, to ICUs, and many floors within the hospital network."
 Assembled face shields ready for distribution. Image courtesy of Nicole Wake, PhD.
Assembled face shields ready for distribution. Image courtesy of Nicole Wake, PhD.The lab has printed 761 of its own 3D-printed face shields and also received additional donations of 5,123 3D-printed shields. Some of these shields are being washed and reused; cleaning instructions have been disseminated on how to clean them, Wake said.
"People really love wearing these and are happy to have them," she said. "So we are continuing to make shields around the clock and distribute them. I think we're close to 5,000 now."
Surgical face masks
Dr. Beth Ripley, PhD, a radiologist and director of the Veterans Health Administration (VHA) 3D Printing Network and chair of the VHA 3D Printing Advisory Committee, has focused on 3D-printed surgical face masks.
 Dr. Beth Ripley, PhD, director of the VHA 3D Printing Network and chair of the VHA 3D Printing Advisory Committee.
Dr. Beth Ripley, PhD, director of the VHA 3D Printing Network and chair of the VHA 3D Printing Advisory Committee.Ripley highlighted the U.S. National Institutes of Health (NIH) 3D Print Exchange as a resource for vetting 3D designs and figuring out which ones adhere to U.S. Food and Drug Administration (FDA) guidelines. Of the 494 total submissions so far to the exchange, 21 have been successfully evaluated, including 12 face shields and five masks, she said.
Four of these masks are community-use designs, while one is a surgical face mask. No designs for 3D-printed N95 respirators have been successfully evaluated on the site, Ripley said.
Notably, the FDA published a new enforcement policy earlier this month for face masks and respirators during the COVID-19 public health emergency. Surgical face masks are considered a medium/low-risk device, which under the emergency measures, requires meeting FDA standards under existing regulations or guidance, Ripley said.
"We learned early on that some of the ASTM standards that you normally have to meet are tests that take months to get back, and we don't have that kind of time," she said. "So we had to work really hard to understand what the risks were and how we could best mitigate them and how we could sort of swap in things in to address those risks."
Assessing mask designs
Ripley said that there are five main risks that must be addressed via standards: breathability, liquid barrier protection, flammability, bacterial filtration efficiency, and particulate filtration efficiency. Although they have since been able to perform the actual tests that needed to be done, they also wanted to perform functional assessments, she said.
For example, they evaluated breathability by having someone wear a mask and perform two minutes of high-quality cardiopulmonary resuscitation (CPR) or run up and down stairs while wearing the mask. Liquid barrier protection was tested by shooting synthetic blood at the masks and filters.
After testing a number of printed mask designs, they weren't able to find one that could meet those standards. So they decided to create their own, Ripley said. Taking into account feedback from surgeons and radiologists, they were able to produce a mask design that was sufficient and meets all of the standards, she said.
"But we're far from done," she said. "There are still a lot of challenges in this space that we still need to address."
For example, filters are a real challenge, Ripley said. The current design of their mask relies on using an existing surgical mask and cutting it into four pieces -- ending up with four masks for the price of one.
"But that is not ideal and we're actively looking for filters," she said. "But the filter challenge comes down to two of the standards. One, you need to be able to breathe through it, and two, it needs to be able to provide liquid barrier protection. So that is an active search that many groups across the country and probably the world are working on."
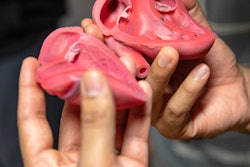
 3D-printed face mask. Image courtesy of Dr. Beth Ripley, PhD.
3D-printed face mask. Image courtesy of Dr. Beth Ripley, PhD.Fitting is also a challenge. A cloth mask has a little more give and room for a better fit than a hard 3D-printed mask, she said. The group is also working on a new version of the mask to make it easier to breathe through.
Nasopharyngeal swabs
The inability to perform enough testing has been a big hurdle to the medical response to COVID-19 in the U.S. Unfortunately, nasopharyngeal swabs are produced in northern Italy and China, according to Summer Decker, PhD, director for 3D clinical applications in the department of radiology at USF Health Morsani College of Medicine in Tampa, FL.
"So there was immediately a global supply chain disruption when [COVID-19] took off in Asia and Europe," Decker said. "That made it very difficult for the U.S. to be able to respond."
 Summer Decker, PhD, director for 3D clinical applications at USF Health Morsani College of Medicine.
Summer Decker, PhD, director for 3D clinical applications at USF Health Morsani College of Medicine.Decker shared how a collaboration between USF Health and Northwell Health has enabled 3D printing of these vital testing swabs. The team was spearheaded by Decker; technical director Jonathan Ford, PhD, of USF; and Todd Goldstein, PhD, of Northwell Health.
The team also contacted FormLabs because they were using that company's printers, and they worked with the company's legal department to determine how this information could be publicly disseminated, Decker said. Team members included representatives from labs and physicians from a variety of specialties, including radiology, infectious disease, emergency medicine, and ear, nose, and throat (ENT).
Swab design process
Initially, the team members came up with about 12 different designs for what might work to get a sufficient sample and be comfortable for patients. When considering what printers and materials to use, they realized that FormLabs 3D printers were widely available, were not as expensive as other printers, and also had FDA-cleared surgical guide material. The surgical guide material could be immediately used for the swabs because it could be autoclaved, or sterilized, Decker said.
A number of mock-ups were printed and provided to infectious disease physicians, ENT or otolaryngology physicians, and pulmonary radiologists for feedback. These physicians even volunteered to try the designs on themselves.
"So we had residents and faculty trying them out for patient comfort and safety," she said.
The design has been modified over time, including a change to make it a little softer on the tip, she said. A pediatric version has also been produced.
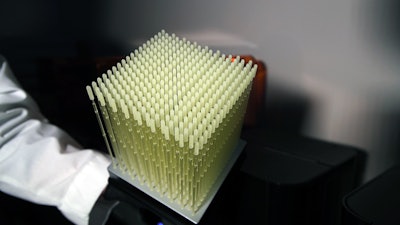 Cube of 3D-printed nasopharyngeal swabs. Image courtesy of Summer Decker, PhD.
Cube of 3D-printed nasopharyngeal swabs. Image courtesy of Summer Decker, PhD.Decker said that the team then worked with FormLabs to figure how many swabs they could print with each batch. They can now print 324 swabs in each batch using the company's surgical guide resin.
Depending on the printer model used, printing time varies from 15 to 30 hours. The team is currently testing a new method that will decrease the printing time for the printer that currently requires 30 hours, Decker said.
After the swabs have been printed, they are cured and then delivered to the infectious disease labs in the hospital. Each swab is then individually wrapped and autoclaved based on sterilization guidelines provided by FormLabs.
Finally, the swabs are bundled with the other testing kit components -- the test tube and the viral transport media -- and then handed off to the different institutions and affiliates that each 3D printing lab is able to print for, she said.
Clinical trials
After bench lab testing of the initial design showed that the 3D-printed swab actually performed as well as (in some areas, better than) the traditional swab, the team quickly launched a clinical trial.
In a study of 120 patients at Northwell and Tampa General Hospital (TGH), a synthetic swab and 3D-printed swab were inserted into the same patient in alternating nostrils. The tests agreed in 113 (94%) of the patients. Of the seven discrepancies, two had synthetic results that were negative but came back as positive on the 3D-printed swabs. Five results were inconclusive.
Fifty-four results were positive for COVID-19, and the cycle threshold (Ct) values were statistically the same. Both sites had similar data and found that the 3D-printed swab was as good as the synthetic swab, Decker said. The team plans to publish the final results of the trial as the other hospitals participating in the multisite clinical trial finish reporting their data.
The 3D-printed swabs are now being used in hospitals. As of now, TGH has 17,000 swabs, Moffitt Cancer Center has 4,500 swabs, and USF Health has 3,000 swabs. In addition, Northwell Health is using 25,000 swabs per week, Decker said.
Each swab has a materials cost of approximately 25¢. Until they are used, the swabs are stored in a dark location. Per instructions from FormLabs, the team is currently using 30 days as an expiration date.
Decker noted that the swab designs have been patented, but they are available for noncommercial purposes until April 15, 2021. The team has also signed a noncommercial agreement with FormLabs to enable the company to print the swabs at their print farm, she said.
Institutions who have any questions about the swabs or are interested in becoming a test site can contact the team. In addition, those interested in procuring the swabs themselves can reach out to FormLabs.