In patients with early-stage breast cancer, "signatures" derived from MRI radiomics and patient clinical risk characteristics can be utilized to preoperatively predict axillary lymph node metastasis as well as disease-free survival, according to research published online December 8 in JAMA Network Open.
In a retrospective analysis, a team of researchers from China developed two clinical-radiomic nomograms that yielded a high degree of accuracy for predicting axillary lymph node metastasis status and performing risk stratification in these patients.
"The clinical-radiomic nomograms were useful in clinical decision-making associated with personalized selection of surgical interventions and therapeutic regimens for patients with early-stage breast cancer," wrote the authors, led by Dr. Yunfang Yu of Sun Yat-sen Memorial Hospital in Guangzhou.
In breast cancer, the presence of axillary lymph node metastasis strongly affects a patient's prognosis for recurrence. However, sentinel lymph node biopsy -- the commonly used technique for assessing this status -- is invasive and subject to a high false-negative rate.
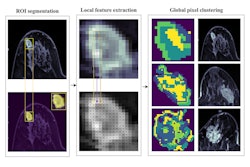
In addition, there is a lack of accurate, preoperative noninvasive methods for predicting axillary lymph node metastasis status and disease-free survival, according to the authors. As a result, the researchers sought to develop and validate dynamic contrast-enhanced MRI radiomic signatures for these tasks.
They retrospectively gathered data from 1,214 women with histologically confirmed early-stage breast cancer from four hospitals in China for the time period of July 3, 2007, to September 21, 2019. Of these, 70% were used for the development cohort and 30% were utilized as the validation cohort. These patients, who had all received preoperative MRI exams, were treated with surgery and sentinel lymph node biopsy or axillary lymph node dissection.
The researchers developed an MRI radiomics signature for axillary lymph node metastasis and disease-free survival predictions by using machine-learning algorithms to analyze extracted radiomics features. This signature yielded an area under the curve (AUC) of 0.88 in the development cohort and 0.85 in the validation cohort for identifying axillary lymph node metastasis. It also predicted three-year disease-free survival at an AUC of 0.81 in the development group and 0.73 in the validation group.
Next, the researchers developed two clinical-radiomics nomograms that incorporate analysis of clinical risk factors -- a clinical signature -- along with the radiomics signature. One nomogram predicts axillary lymph node status while the second provides predictions for disease-free survival.
| Performance of clinical/radiomics nomograms in early-stage breast cancer | ||
| Development cohort | Validation cohort | |
| Predicting axillary lymph node metastasis (AUC) | 0.92 | 0.90 |
| Discriminating high-risk and low-risk patients (hazard ratio) | 0.04 | 0.04 |
| Association with 3-year disease-free survival (AUC) | 0.89 | 0.90 |
The researchers also reported that decision-curve analysis showed that the clinical-radiomic nomogram displayed better clinical predictive usefulness than the clinical or radiomic signatures alone.
Although the study represents a promising step forward toward radiomics-based surgical guidance, more work is required before these types of tools will be ready for clinical use, according to Nathaniel Braman, PhD, of Case Western Reserve University.
It will be crucial to confirm its performance using data unseen during training, including on different scanners and acquisition protocols, Braman said in an accompanying editorial. Performance should also be evaluated among international populations.
What's more, clinical adoption will require replacing the current manual image annotation step with either a fully automated, high-performing, and generalizable segmentation approach or an in-depth analysis of the impact on performance caused by inter-reader annotation variability, Braman said. He noted that a final model will also need to be selected based on new data.
"Finally, it will ultimately be critical that the signature be prospectively validated for guiding surgical approach and compared with [sentinel lymph node biopsy] for improved outcomes," Braman wrote. "The retrospective performance of radiomic decision-support tools will ultimately be immaterial unless they can also yield tangible improvements to quality of care for patients whose treatment has reached a crossroads."