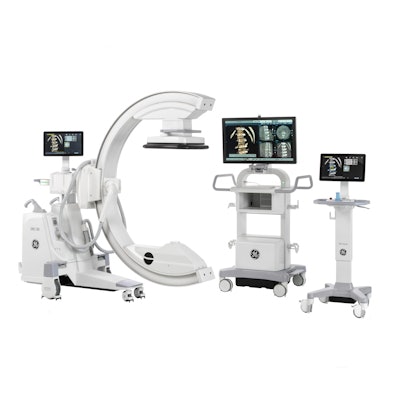
The U.S. Food and Drug Administration (FDA) has cleared GE Healthcare's new orthopedic surgery imaging system, OEC 3D. The C-arm performs both 2D and 3D imaging.
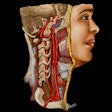
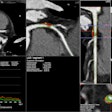
The system is capable of producing volumetric images for spine and orthopedic procedures, the company said. OEC 3D is built on GE's OEC Elite C-arm platform, and it boasts a 19 x 19 x 19 cm field-of-view display. It also features GE's Volume Viewer for image display and surgical assessment, according to the firm.
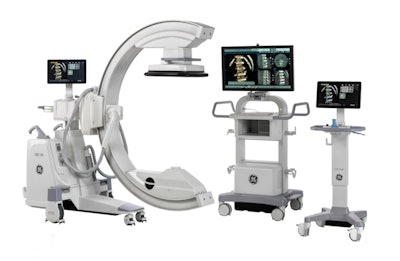 GE's new OEC 3D surgical C-arm. Image courtesy of GE Healthcare.
GE's new OEC 3D surgical C-arm. Image courtesy of GE Healthcare.Clinicians who used the C-arm in clinical evaluations reported that the image quality of the 3D volume is similar to what they would expect from a CT scanner, according to GE. Users also reported that it was easier to have just one C-arm that was capable of performing both 2D and 3D imaging, rather than two separate systems.