The U.S. Food and Drug Administration has cleared the CloudVue mobile 3D cinematic rendering software from International Medical Solutions (IMS).
CloudVue allows radiologists and other healthcare professionals to access, view, and share photorealistic cinematic rendered medical images in the cloud using any device. It does not require any on-premises back-end servers, according to the company.
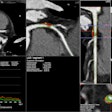
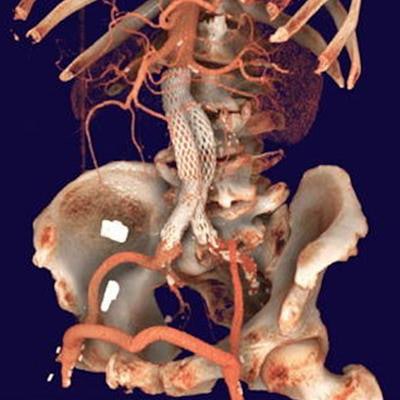
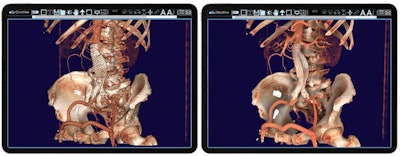 Using IMS' CloudVue viewer for mobile 3D with cinematic rendering, physicians can use both views to determine the procedure's efficacy. One can see the stent placement in both environments, and mobile 3D with cinematic rendering. The surgical team is using these images to determine the outcome of this patient's procedure. Image courtesy of IMS.
Using IMS' CloudVue viewer for mobile 3D with cinematic rendering, physicians can use both views to determine the procedure's efficacy. One can see the stent placement in both environments, and mobile 3D with cinematic rendering. The surgical team is using these images to determine the outcome of this patient's procedure. Image courtesy of IMS.The company notes that the software only requires one central processing unit (CPU) core to support up to 30 users to view high-quality cinematic image renderings with the same performance as a back-end server.
What's more, clinicians can access cinematic-rendered images in the cloud on any device (desktop, laptop, smartphone, or tablet) with no cloud latency or app installs required.