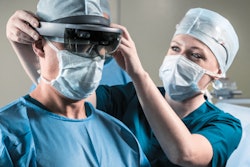
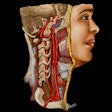
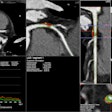
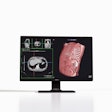
The U.S. Food and Drug Administration (FDA) has given 510(k) clearance to ImmersiveTouch for its modular software that allows users to import, visualize, and automatically segment medical images to create 3D representations.
The software will reduce planning hours and increase efficiency as the models can be used for medical diagnosis, anatomical measuring, treatment planning, and generating output files for additive manufacturing, the firm said.