Printing and IT services provider Ricoh has secured 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its craniomaxillofacial and orthopedic anatomic modeling.
Ricoh's 3D for Healthcare is an end-to-end workflow platform that develops, designs, and produces 3D-printed anatomic models using technology from 3D printing technology firm Stratasys.
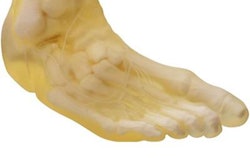
Integrated with IBM iConnect Access, 3D for Healthcare creates patient-specific representations of tissue and bone. The models are meant to serve as physical simulators that help clinicians see inside anatomy but are not intended for diagnosis or treatment.
Providers can choose a point-of-care option in which a Ricoh managed services team manages the process using Stratasys 3D printers, or they can select an on-demand option in which they order and have the models printed at a Ricoh facility and shipped directly to them, the company said.