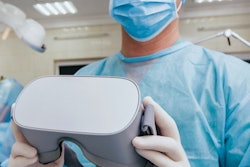
St. Louis-based augmented reality (AR) developer SentiAR is highlighting that the company has received its second 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its CommandEP system.
CommandEP integrates existing 3D cardiac mapping systems to create a real-time 3D holographic interface. This provides physicians with an interactive, 360-degree view of the patient's specific anatomy to allow them to deliver cardiac ablation therapy.
The system uses a specially designed headset worn by the physician. This allows for hands-free manipulation of the patient's data, in addition to real-time heart anatomy and catheter locations inside the heart. This technology provides physicians with control of their digital tools during cardiac ablation procedures.
The company also said it anticipates completion of new clinical studies later in 2023.