Brachytherapy firm Cianna Medical has received clearance from the U.S. Food and Drug Administration (FDA) for its Strut-Adjusted Volume Implant (SAVI) Scout surgical guidance system.
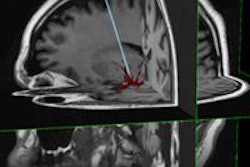
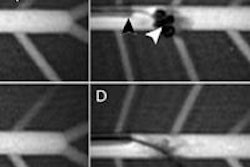
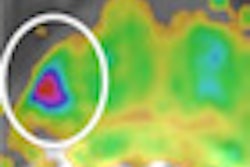
SAVI Scout is designed with real-time audible and visual indicators to help surgeons target specific tissue during lumpectomy and excisional biopsy procedures. The system uses electromagnetic wave technology to detect a reflector that can be placed in the target tissue up to seven days prior to surgery, the company said.
During the procedure, the surgeon uses SAVI Scout to locate the reflector and plan the incision. The surgeon then removes the reflector and the target tissue.