Advanced visualization software developer EDDA Technology has received clearance from the U.S. Food and Drug Administration (FDA) for its IQQA-Guide technology.
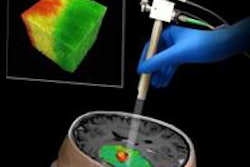
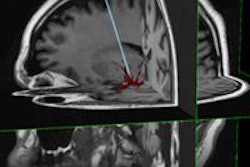
IQQA-Guide is a package of hardware and software designed to provide real-time 3D image guidance in the interventional suite for abdominal and thoracic biopsy, interventional procedures, and surgery, EDDA said.
IQQA-Guide utilizes a 3D map of individualized anatomic representations from multimodality images, providing real-time tracking and referencing to anatomic features of interest in 3D.