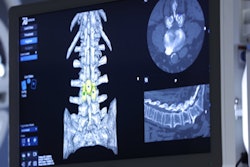
7D Surgical has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and a medical device license from Health Canada for 7D Surgical System, its machine-vision image-guided surgery (MIGS) system for spine surgery.
7D Surgical System uses 3D optical technologies and machine-vision algorithms, registering spinal surgery patients automatically using only visible light. The system also uses the company's Flash Registration of the patient's anatomy, meaning the workflow takes less than 20 seconds for de novo spinal registration.
The product's MIGS technology is embedded in an onboard overhead surgical light, which eliminates line-of-sight frustrations in the operating room, and the software is controlled by the surgeon using only a foot pedal, 7D Surgical said.
The firm has started its North American commercialization strategy since receiving its clearances.