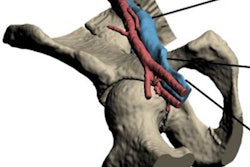
With the availability of a 3D printing material that's detectable on both CT and MRI, radiologists can simulate and practice complex image-guided cryoablation of head, neck, and spinal tumors using 3D-printed models, according to research presented at RSNA 2017.
Researchers from the U.S. and Canada mixed different combinations of 3D printing materials visible on CT and MRI to create individually tailored 3D-printed spinal models. They used these models to simulate CT-guided needle placement and MRI-guided monitoring for the cryoablation of a spinal tumor in two separate patients.
"For the first time, we can create 3D-printed models that replicate in vivo patient-specific anatomy on both CT and MRI," Dimitris Mitsouras, PhD, director of the Applied Imaging Science Laboratory at Brigham and Women's Hospital, told AuntMinnie.com. "This type of technology, if it confers to these procedures the same advantages as 3D printing does for open surgical procedures, would be a significant leap forward."
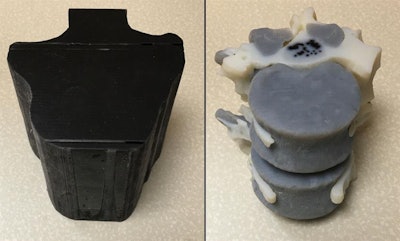 3D-printed spinal model visible on CT and MRI (left). Internal structure of the model (right). All images courtesy of Dimitris Mitsouras, PhD.
3D-printed spinal model visible on CT and MRI (left). Internal structure of the model (right). All images courtesy of Dimitris Mitsouras, PhD.A pressing need
In recent years, physicians have been using patient-specific 3D-printed models to simulate or assess a wide range of intricate surgeries -- including those of the hip, aortic valves, and kidneys. The various benefits of planning and practicing complex surgeries on 3D-printed models include a reduction in complications, more efficient use of resources, and improved patient outcome.
Image-guided interventions account for a considerable portion of radiology procedures performed, and they are becoming increasingly more common because of their minimally invasive nature, Mitsouras said. But creating 3D-printed models fit for planning image-guided procedures is not an easy task.
 Dimitris Mitsouras, PhD, from Brigham and Women's Hospital.
Dimitris Mitsouras, PhD, from Brigham and Women's Hospital."The 3D models that must be created from patient images need to not only spatially display patient anatomy and pathology as standard 3D anatomic models do, but they also need to replicate the different radiographic properties of the different tissues in the imaging modalities that are used to guide the procedures," he said.
Catering to multiple imaging modalities complicates "the main limitation of creating models for planning image-guided procedures: a lack of materials with sufficient differences in x-ray attenuation," presenter Dr. Elizabeth George told session attendees.
Specifically, for CT, the density of typical 3D printing materials is restricted to a narrow range of Hounsfield units (about 25 to 90). In the case of MRI, there is only a single photopolymer capable of generating an adequate signal, which Mitsouras and colleagues recently identified in a previous 2016 study.
Using this photopolymer, the researchers sought to fulfill the pressing need to produce 3D-printed models for the simulation and training of complex CT- and MR-guided therapies. The need was particularly evident in two patient cases in which a residual tumor sat adjacent to the spinal nerves and thecal sac, requiring spinal cryoablation.
"This project was largely driven by the need to simulate spinal cryoablations for two patients with osteoblastomas at our institution to ensure safety of the nerves," George said. "These procedures are performed under CT and MRI guidance for drilling to the tumor and monitoring the ablation zone, respectively."
'Biomimicking' models
The researchers began creating the 3D-printed spinal models for the two patients with osteoblastomas by segmenting previously acquired images. They segmented the cortical bone, bone marrow, osteoblastoma, diaphragmatic crura, and aorta from prior CT scans; the bone marrow, crura, aorta, epidural and paraspinal fat, thecal sac, and spinal nerves from T2-weighted MR images; and the bone marrow and spinal nerve roots from T1-weighted MR images.
Next, the researchers combined the segmented portions of the CT scans with the segmented components of the MR images by aligning the identical structures found in both modalities, i.e., the bone marrow, crura, and aorta. They input this composite 3D model into a 3D printer capable of creating models using two different materials (Objet500 Connex 2, Stratasys).
The two base materials were a black rubber material with very low CT signal (FLX980) and a high-temperature material with medium MR signal and high CT signal (RGD525). Mixtures of these two materials provided individual tissues with distinct compositions visible on CT and MRI. The 3D printer encased the spinal model in a shell and filled the empty areas of the shell -- representing the fat tissue and cerebrospinal fluid -- with a glycerin-based support material.
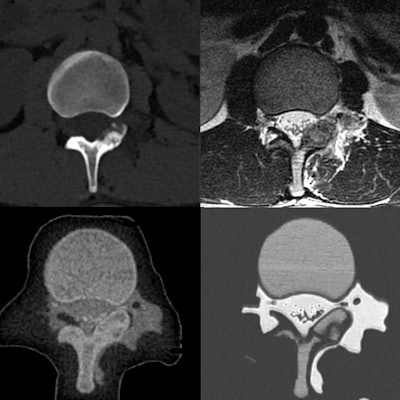
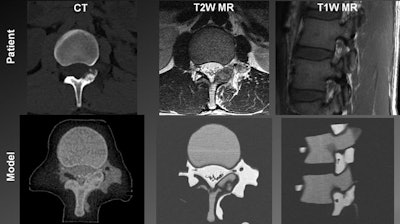 Top from left to right: Patient spine visualized in a CT scan, a T2-weighted MR image, and a T1-weighted MR image. Bottom from left to right: 3D-printed spinal model visualized in a CT scan, a T2-weighted MR image, and a T1-weighted MR image.
Top from left to right: Patient spine visualized in a CT scan, a T2-weighted MR image, and a T1-weighted MR image. Bottom from left to right: 3D-printed spinal model visualized in a CT scan, a T2-weighted MR image, and a T1-weighted MR image.After 3D printing, the researchers planned and then simulated the CT-guided power drilling and MRI-monitored cryoablation procedure on a spinal model in a surgical suite. They were able to monitor the ice-ball expansion step of the cryoablation in T2-weighted MR images of the model as well as they would have been able to in MR images of an actual patient.
A comparative analysis of images of the 3D-printed spinal model and patient anatomy revealed that all relevant tissues that are normally distinguishable on in vivo CT scans and MR images were also discernible on the scans and images of the spinal model. Each mixture of "biomimicking" materials also had a high enough contrast-to-noise ratio (greater than 5:1) to be sufficiently distinguishable on CT and MRI.
Paving the way
Moving forward, more research is needed to assess the precise shape and extent of the ablation zone, George said. For this, manufacturers may need to develop 3D-printed materials that match the tissue and thermal properties and image characteristics of MR relaxation rates and CT Hounsfield units even better.
 Dr. Elizabeth George from Brigham and Women's Hospital.
Dr. Elizabeth George from Brigham and Women's Hospital.For now, training on 3D-printed models has contributed to the successful cryoablation of spinal osteoblastomas in two young patients, without leaving residual tumor or causing neural damage, according to Mitsouras. Using this technique may further optimize the safety and efficacy of ablation procedures, as well as allow radiologists to try different approaches to maximize yield and minimize complications.
"The most direct implication of this [technique] is the enormous opportunities it opens up for image-guided procedure simulation and training," he told AuntMinnie.com. "Without these models, a very large body of experience is absolutely necessary to guide such choices."
A key implication of using these or similar biomimicking models is that they may facilitate diagnostic and interventional radiology research, he said. One can potentially create biomimicking 3D models representing different stages and variants of a disease to optimize new CT- and MR-guided methods as well as test the impact of lowering CT radiation dose on diagnostic accuracy.
"We hope this technology will begin to pave the way for interventional radiologists to be able to practice and simulate challenging procedures for specific patients, to assess the safety and accuracy of their treatment plan, and, importantly, to provide hands-on training for residents," he said.