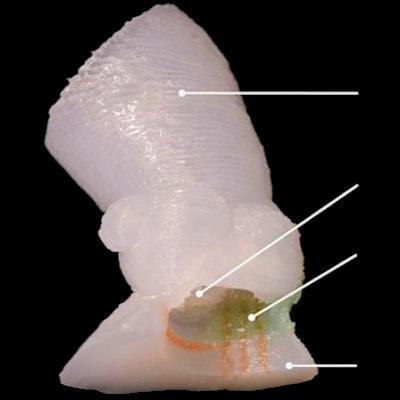
3D-printed soft aortic root models with integrated electronic sensor arrays can improve the planning of transcatheter aortic valve replacement (TAVR) procedures in patients with aortic stenosis, potentially decreasing the risk of complications, according to research published online August 28 in Science Advances.
Using a customized 3D printing process and specialized inks, a team of researchers from the University of Minnesota has developed a new process for producing patient-specific aortic root models from CT scans. These models, which are able to mimic soft tissue and the hard calcification on the valve flaps, include integrated 3D-printed sensors that can help in determining the size and placement of the valve device during the TAVR procedure.
"These models may pave exciting avenues for mitigating the risks of postoperative complications and facilitating the development of next-generation medical devices," wrote the authors led by Ghazaleh Haghiashtiani, PhD, and Kaiyan Qiu, PhD. Scientists from interventional technology firm Medtronic also participated in the research project.
TAVR procedures can result in postoperative complications such as paravalvular leak and conduction disturbances. Proper selection and optimization of the interplay among the patient's anatomy, the procedure, and the bioprosthetic valve are vital to mitigate the risk of complications and mortality, according to the researchers.
Although commercial 3D printers can print the shape of the aortic root models, they typically use inks that are often too rigid to match the softness of real heart tissue, the researchers said. As a result, the authors turned to specialized 3D printers and silicone-based inks that mechanically match the feel of real heart tissue that was obtained from the university's Visible Heart Laboratories. Specifically, the models utilized three different materials with properties that were comparable to the aorta, myocardium and leaflets, and calcified regions.
Thanks to the integrated, 3D-printed electronic sensor arrays within the model, physicians also receive electronic pressure feedback that can help to guide and optimize the selection of the valve within the patient's anatomy, according to the group.
"These models can be applied for TAVR preprocedural planning and potentially inform prosthetic device sizing and procedure parameters, patient- and disease-specific hemodynamic assessment, and possible [paravalvular leak] pathways," the team wrote. "In addition, these models may provide clinicians with a comprehensive benchtop tool for understanding the deployment of the valve and mitigating some of the risks of post-TAVR conduction disturbances."
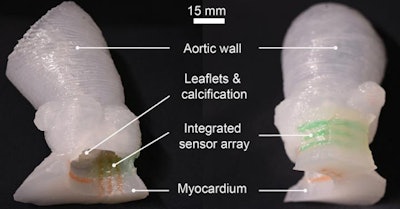 Patient-specific 3D-printed aortic root models with integrated 3D-printed soft sensor arrays can be used to improve planning of TAVR procedures. Images courtesy of the McAlpine Group, University of Minnesota.
Patient-specific 3D-printed aortic root models with integrated 3D-printed soft sensor arrays can be used to improve planning of TAVR procedures. Images courtesy of the McAlpine Group, University of Minnesota.The efficaciousness of their approach was demonstrated by comparing the models with postoperative patient data, as well as by in vitro hemodynamic performance testing, according to the researchers.
In addition to aiding in surgical planning, the models could also be used to help patients better understand the procedure and their own anatomy, according to senior author Michael McAlpine, PhD, of the University of Minnesota
"As our 3D-printing techniques continue to improve and we discover new ways to integrate electronics to mimic organ function, the models themselves may be used as artificial replacement organs," McAlpine said in a statement. "Someday maybe these 'bionic' organs can be as good as or better than their biological counterparts."