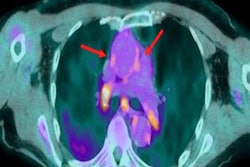
PET imaging has shown how impaired blood flow in small vessels of the heart may lead to myocardial stress and injury in patients with aortic stenosis, according to a study published online September 15 in JAMA Cardiology.
The U.S. researchers, led by senior author Dr. Marcelo Di Carli of Brigham and Women's Hospital in Boston, used cardiac PET to analyze myocardial flow reserve in patients with aortic stenosis and compared it with patients without aortic stenosis. The team found that PET may serve as an early sign for identifying which patients are at higher risk for heart failure.
"[Myocardial flow reserve] may serve as an early sensitive marker of myocardial decompensation to identify patients with [aortic stenosis] who are at high risk," the team wrote.
Aortic stenosis is the narrowing of a patient's aortic valve, which causes increased stress in the myocardium. Patients with severe cases are at risk of heart failure and have low survival rates without aortic valve replacements.
Cardiac PET can be used to assess myocardial flow reserve, a measure of the ability of the heart's small blood vessels to increase blood flow under stress. Previous studies by Di Carli and colleagues have shown that impaired myocardial flow reserve is associated with adverse left ventricular remodeling (for instance, the thickening of the heart wall) and worse clinical outcomes in patients with aortic sclerosis.
Those studies were conducted in patients mostly with aortic sclerosis and mild disease, however. In this prospective observational study, the group sought to evaluate myocardial flow reserve on cardiac PET in patients with more severe disease.
The researchers enlisted 34 individuals with moderate to severe aortic sclerosis and 34 control individuals to participate in the study. Between 2018 and 2020, the patients underwent nitrogen-13-labeled ammonia PET myocardial perfusion imaging, resting transthoracic echocardiogram, and serum biomarker collection at Brigham and Women's Hospital in Boston.
A subset of patients underwent repeated assessment six months after aortic valve replacement.
The study authors found that myocardial flow reserve was independently associated with global longitudinal strain (GLS) and left ventricle ejection fraction. Stress myocardial blood flow was associated with high-sensitive cardiac troponin T (hs-cTnT) and N-terminal pro hormone B-type natriuretic peptide, two standard clinical biomarkers for heart failure.
The combination of low-stress myocardial blood flow and high hs-cTnT was associated with higher interventricular septal thickness in diastole, relative wall thickness, and worse GLS compared with high-stress myocardial blood flow and low hs-cTnT, the researchers found.
"Myocardial flow reserve was associated with adverse myocardial characteristics and stress myocardial flow reserve was associated with markers of myocardial stress and injury," they stated.
In addition, in nine patients studied six months after valve replacement surgery, mean myocardial flow reserve improved from 1.73 to 2.11, which further suggests myocardial flow reserve may be an early sensitive marker for myocardial decompensation, Di Carli and colleagues suggested.
The authors noted limitations, namely that the study was a single-center observational study with a relatively small sample size and that many of the patients had hypertension, which can reduce myocardial blood flow.
"Future studies are needed to investigate whether [myocardial flow reserve] alone or in combination with biomarkers of myocardial wall stress and injury may identify patients who might benefit from earlier valvular intervention, especially in those with moderate [aortic stenosis] or asymptomatic severe [aortic stenosis]," they concluded.