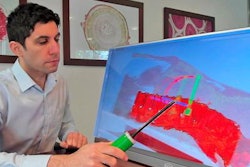
Austrian firm Interventional Systems has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Micromate medical robot.
Designed to perform almost every noninvasive image-guided interventional procedure, the table-mounted medical robot utilizes a universal instrument guidance system that enables physicians to continue using their preferred surgical instruments, according to the vendor. Targeting is performed under live imaging using any conebeam CT, CT fluoroscopy, or fluoroscopy system, Interventional Systems said.
The company now plans to initiate operations in the U.S. in the second half of 2021 by launching centers of excellence for robotic-guided interventional procedures. In addition, Interventional Systems said it will establish commercial partnerships with third parties.