Imaging processing developer Clario Medical Imaging has received 510(k) marketing clearance from the U.S. Food and Drug Administration for its z3D Contrast Acuity software.
The application will be initially distributed to customers through a license agreement with Confirma and Invivo, according to Bellingham, WA-based Clario.
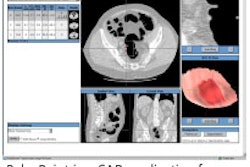
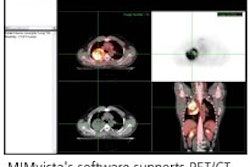
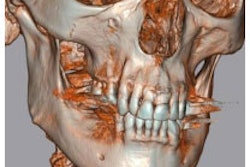
The z3D software is used to analyze multimodality digital images, including MR, CT, x-ray, PET, and nuclear medicine images the company said.
By AuntMinnie.com staff writers
December 14, 2005
Related Reading
Clario teams up with Confirma, Invivo, November 23, 2005
Copyright © 2005 AuntMinnie.com