Advanced visualization software developer Ziosoft has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its MR cardiac function analysis application.
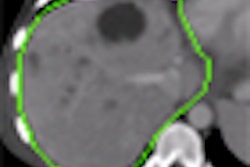
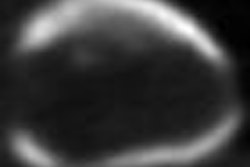
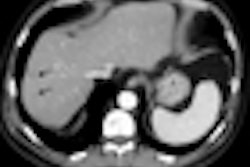
The module, available on Ziosoft's Ziostation thin-client advanced visualization software, is used to evaluate heart structures and function during the initial screening and subsequent management of coronary disease patients, according to the Redwood City, CA-based vendor. It can extract left ventricular myocardial contours from Ziosoft's automatic interpolation algorithm, Ziosoft said.
Other capabilities include automatic calculation of myocardial mass, as well as standard assessment calculations such as ejection fraction, peak filling rate, and peak ejection rate, the company said.
Related Reading
Ziosoft nets CE Mark, July 1, 2009
FDA clears Ziosoft cardiac software, April 9, 2009
Ziosoft rolls out Ziostation program, March 10, 2009
Ziosoft opens European office, March 3, 2009
Ziosoft gets software OK, January 7, 2009
Copyright © 2009 AuntMinnie.com