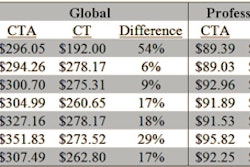
Postprocessing services provider Precision Image Analysis (PIA) is expanding its capabilities to provide medical imaging services.
Building on its ISO-9001:2008 certification for its Quality Management System, PIA has adopted and implemented comprehensive Core Lab protocols and procedures. This enables PIA to support a wide variety of research activities. Its Core Lab services include design and optimization of protocols, intraobserver and interobserver analyses, statistical analysis, and customized reporting.
In addition, PIA has built a system compliant with Title 21 CFR Part 11 that applies to medical device manufacturers, drug makers, biotech companies, biologics developers, contract research organizations, and other industries regulated by the U.S. Food and Drug Administration. Specifically, the system is for companies that are required to implement controls, including audits, system validations, audit trails, electronic signatures, and documentation for software systems involved in processing electronic data.