The U.S. Food and Drug Administration (FDA) has granted an expedited access pathway (EAP) designation to MedyMatch's artificial intelligence (AI) software for detecting intracranial hemorrhage, paving the way for a priority regulatory review as part of the FDA's new Breakthrough Devices program.
Launched in 2015, the EAP designation was created by the FDA for medical devices that demonstrate the potential to address unmet medical needs for life-threatening or irreversibly debilitating diseases or conditions. All EAP devices will soon be transitioned, however, to the FDA's new Breakthrough Devices program, which was created as part of the 21st Century Cures Act. Building on the EAP program, the Breakthrough Devices program aims to give patients more timely access to devices and technologies that provide treatment or diagnosis for life-threatening or irreversibly debilitating diseases for which no approved or cleared treatment exists, or that offer significant advantages over existing or cleared alternatives, according to the FDA.
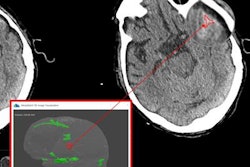
Based on deep-learning techniques, MedyMatch's AI software automatically analyzes noncontrast head CT images for the presence of intracranial hemorrhage, an important finding during the assessment of stroke and head trauma, according to the company. As a new category of medical device, the software will still need to go through the premarket approval (PMA) process. However, the FDA's designation as a breakthrough device will bring access to additional FDA resources, as well as a priority review.
"It's more attention and support from the FDA, as well as working very collaboratively around an expedited pathway to bring this to market sooner," said chairman and CEO Gene Saragnese. "We are absolutely thrilled we could get this designation and that we can partner with the FDA to bring this technology and application to the marketplace."