Artificial intelligence (AI) software developer MaxQ AI has filed papers with the U.S. Securities and Exchange Commission (SEC) for an initial public offering of just over $8 million. The company plans to use the funds to commercialize its Accipio family of AI algorithms for analyzing head CT scans to rule out stroke.
Formerly known as MedyMatch Technology, MaxQ AI filed an 8-K registration statement with the SEC on August 9 to sell shares of stock worth $8.1 million. In the 8-K filing, the firm describes its initial focus as the development of applications for the emergency room and acute care environments based on its proprietary AI platform.
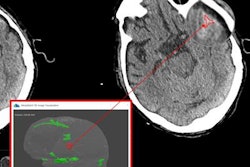
The company's initial family of products, called Accipio, uses machine vision and deep-learning technologies to analyze noncontrast CT scans and help physicians rule out intracranial hemorrhage (ICH) caused by stroke or head trauma.
The company has three main product lines in the Accipio family:
- AccipioDx, designed to support physicians in ruling out intracranial hemorrhage in acute care settings
- AccipioIx, which prioritizes cases of ICH for faster interpretation by readers
- AccipioAx, designed to help clinicians interpret head CT scans by creating a duplicate annotated set of images and a full 3D brain rendering to supplement original, unaltered CT scans
The filing noted that the company so far has not generated any revenue. While its AccipioIx product received the CE Mark in Europe in May 2018, none of the company's other products have been approved for sale yet, and the firm will not be able to generate revenue until it receives those approvals. What's more, MaxQ AI had a $9.2 million loss in fiscal 2017 and a $1.9 million loss in the first quarter of fiscal 2018, and its auditors have expressed doubt about the company's ability to continue as a going concern unless it raises debt or equity financing.
On the positive side, the company has agreements with two major radiology vendors, GE Healthcare and IBM Watson, to integrate its products into their software. It also has a deal with Samsung NeuroLogica to integrate its applications with the company's portable CereTom CT scanners for use in intensive care units, as well as an agreement with Intel to optimize its artificial intelligence technology.
Another recent milestone was the designation of AccipioDx as a breakthrough device by the U.S. Food and Drug Administration (FDA). The breakthrough program is designed to reduce time to market for products by promoting earlier and more interactive engagement with FDA staff during the development and review of medical devices. While no Accipio products have been submitted for FDA approval yet, the company expects to do so in the third quarter of 2018.
MaxQ AI's competitive strengths include its proprietary AI technology that can analyze a wide range of complex 3D medical images, its breakthrough device designation, and its access to market via relationships with OEMs. Other advantages include its access to large, nonpublic catalogs of clinical exams for verifying its technology, as well as an experienced management team led by former GE Healthcare and Philips Healthcare executive Gene Saragnese, who serves as chairman and CEO of the company.
Based in Tel Aviv, Israel, MaxQ AI plans to list its shares on the Nasdaq Capital Market under the symbol MQAI.