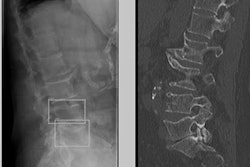
The U.S. Food and Drug Administration (FDA) has cleared Gleamer's BoneView artificial intelligence (AI)-based software, which detects bone fractures on x-rays.
BoneView detects fractures in x-rays and presents them to radiologists for final validation. The software's algorithm is cleared as a computer-assisted detection and diagnosis (CADe/CADx) application and highlights regions of interest with bounding boxes around areas where fractures are suspected so radiologists can prioritize reading those x-rays.
The software is currently available in the U.S. through Gleamer, Fujifilm Medical Systems, Aidoc, Ferrum Health, and Blackford Analysis. BoneView is already being used in 13 countries in Europe, Asia-Pacific, and North America.