Quibim has closed a $50 million series A financing round to support strategic collaborations with hospitals and pharmaceutical companies in the U.S.
The company has been developing human digital twin technology at the organ and lesion level, as well as foundational AI models designed to extract actionable insights from MRI, CT, and PET scans. However, as the volume of whole-body scans and related imaging data grows, in the coming years Quibim models will analyze the entire body, the company noted.
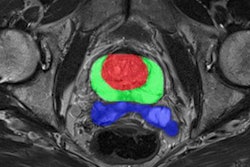
In its announcement, Quibim highlighted its QP-Prostate, QP-Brain, QP-Liver, and QP-Insights, which are cleared for use in the European Union and U.K. through the CE and UKCA marks, some of which are 510(k) cleared by the U.S. Food and Drug Administration (FDA).
Led by Asabys and Buenavista Equity Partners, the funding round includes support from UI Investissements, GoHub Ventures, Amadeus Capital Partners, APEX Ventures, Partech, Adara Ventures and Leadwind, and individual investors.