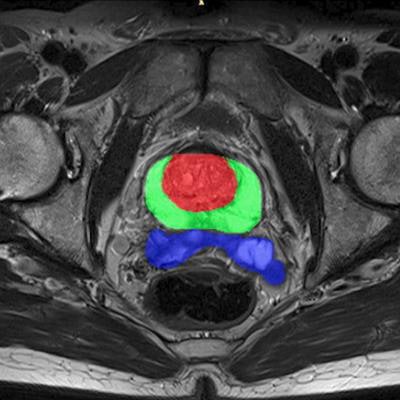
Quibim's qp-Prostate artificial intelligence software for analyzing prostate MRI images has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
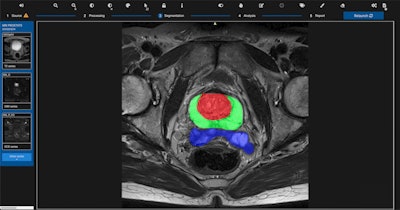 Quibim's qp-Prostate software. Image courtesy of Quibim.
Quibim's qp-Prostate software. Image courtesy of Quibim.The imaging processing software product runs on off-the-shelf workstations, providing visualization and analysis of DICOM data to trained professionals. It enables automated segmentation of regions of the prostate, including the transitional zone, peripheral zone, and seminal vesicles, to aid in image interpretation and diagnoses, according to the company, which is based in Valencia, Spain.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)





.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











