Prostate brachytherapy offers convenience and lower cost for cancer patients who clinically qualify for the treatment, but the success of patient outcomes relies on maintaining rigorous quality assurance (QA). Healthcare IT makes this task easier, according to an article published online in Brachytherapy.
The British Columbia Cancer Agency (BCCA), with headquarters in Vancouver, operates the largest prostate brachytherapy program in Canada and one of the largest of such programs in the world. More than 4,000 patients have received treatment at one of five centers since the program began in July 1998.
The program has expanded its team of four radiation oncologists and two medical physicists at one center to 16 radiation oncologists and 20 medical physicists at five centers. In 2010, 450 of 1,600 patients with prostate cancer underwent this treatment (Brachytherapy, June 25, 2012).
Tracking the biochemical outcomes and toxicities of all the patients, as well as managing the program's logistics, is achieved with the rigorous use of automated electronic patient and operational databases. Information systems provide the functionality to manage patient profiles of eligibility, treatment, and post-treatment tracking and outcomes analysis; treatment planning; automated safety checks; and results comparison and individual benchmarking.
The databases, combined with adherence to unified patient eligibility criteria and treatment planning methodology, extensive training and mentorship programs, positive peer-review and performance analysis philosophies, and a culture of safety, make the program work, according to lead author Dr. Mira Keyes, the brachytherapy program's director and a radiation oncologist at the Vancouver Cancer Center. Keyes and colleagues were motivated to describe their QA program in part from a recommendation by the U.S. Nuclear Regulatory Commission (NRC) that quality assurance procedures of prostate brachytherapy programs be publicized.
All the pieces matter
The motto of BCCA's program is "omnis partis referret," meaning "all the pieces matter." The first piece of the program is patient selection. Uniform eligibility criteria have been defined for patients with low- and intermediate-risk disease. Patients with high-risk disease must be enrolled in a clinical trial for consideration.
Another piece, treatment planning, is also rules-based and consistent. Changes in treatment planning are identified and implemented through an established quality assurance process. Postimplant dosimetry and patient follow-up are also consistent and rules-based, managed and tracked by a functional and user-friendly information system.
The databases
The patient database includes demographics and disease characteristics including pretreatment and initial prostate-specific antigen (PSA) measurements, Gleason score, clinical stage, percent positive cores for risk stratification, pathology, pre- and postimplant dosimetric data, clinical and biochemical follow-up of PSA, and toxicity outcomes. It can be accessed by authorized healthcare providers throughout British Columbia and the Yukon, two of Canada's geographically largest and least populated provinces.
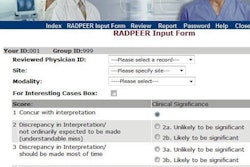
The operational database is a process management system, generating individual task lists for all members of the program for all five sites. Recorded data are presented in a task-related interface, with forms designed with liberal use of drop-down lists to prevent nonsensical values from being entered.
Every task, from importing ultrasound images into the treatment planning system to entering final dosimetric parameters, is recorded and time stamped. The system has also been maximized to transfer data automatically among the tasks it oversees for the various professional disciplines.
While every member of the BCCA Provincial Prostate Brachytherapy Program can access the database, log-ins by individuals trigger data-entry limitations designed to prevent data deletion. Many automated features have been designed to eliminate manual mistakes and typographical errors. Examples include direct importing of dosimetric data from treatment planning software and automatic generation of brachytherapy seed order forms.
When dosimetric parameters and PSA values are entered, these data trigger algorithms that automatically generate lists of cases to review based on preset criteria. If baseline or follow-up patient data are overdue, alert lists are generated.
Brachytherapy seed management is handled on an individual site basis. It includes placing orders for seeds, maintaining inventories, and tracking the number of seeds implanted in a patient, left over, removed from the premises, or destroyed.
Tracking tools monitor patient schedules, sorted by dates, clinics, oncologists, and pending tasks. A dosimetry QA list contains all patients who need QA review, and a PSA flag list identifies patients who need to have manual review of PSA profiles. Patients with postimplant infections are monitored with a specialized tracking tool so that timely and appropriate treatment is provided.
The process management database is designed to continuously update itself. When activities are completed that trigger initiation of another task, new lists and notices are generated, keeping caregivers updated.
A radiation oncology self-assessment summarizes a practitioner's quality assurance results. It contains online, real-time reports on acute and late toxicity of patients, PSA failure rates, and dosimetry information. This is accompanied by benchmark data for comparative analysis. Review of this information is voluntary, but the majority of radiation oncologists access it regularly.
Other program elements
Electronic management is augmented by annual credentialing to ensure that radiation oncologists, dosimetrists, and medical physicists are retaining their clinical skills. Training and mentoring programs are ongoing, and participation in structured continuing education programs is encouraged. Peer-review activities and quality assurance review vary within each treatment facility. Efforts are under way to make these activities more time efficient.
Patient outcomes are assessed and compared against other institutions with brachytherapy programs. Research activities that pertain to patient care are encouraged. Strong leadership, a culture of patient safety emphasizing group participation by all disciplines, and continuous review contribute to what the authors believe is an excellent quality assurance program.
The operational database has been a major part of the success. Its multiple automatic safety checks incorporated into the management of day-to-day operations by numerous individuals has reduced the number of errors to a minimum, the authors wrote.
The sum of these systematic safeguards and individual QA processes has been the cornerstone of the BCCA philosophy.
The support of rigorous brachytherapy QA procedures has contributed to the excellent treatment outcomes for their patients, they concluded.