Spurred by the recent emphasis on reducing radiation exposure, researchers in Boston have developed a way to create a data repository from nuclear medicine reports to better facilitate patient-specific monitoring of radiation dose, according to a study published online May 24 in Radiology.
Led by Dr. Ichiro Ikuta, physicians from Brigham and Women's Hospital and Harvard Medical School developed PARSE -- Perl Automation for Radiopharmaceutical Selection and Extraction -- to extract information from patient reports on radiopharmaceuticals and how they were administrated.
Based on PARSE's data-extracting capabilities, recall (sensitivity) of 97.6% and precision (positive predictive value) of 98.7% were calculated with 95% confidence intervals.
With this information on patient radiation exposure in hand, healthcare providers could further advance patient safety and quality assurance programs designed to monitor operations, limit radiation exposure to reasonable levels for patients, and provide data for patient-specific radiation dose estimates.
Patient safety
Previous research has found that CT accounts for approximately 49% of patient exposure to ionizing radiation, while nuclear medicine accounts for approximately 26%, according to the authors.
"For the medical community to engage in systematic and large-scale patient safety initiatives related to radiation exposure, it must first have the tools to capture radiation exposure data on a large scale, not only for CT, but for nuclear medicine as well," they wrote.
The researchers reviewed the records of inpatient, outpatient, and emergency department visits at Brigham and Women's and its affiliated facilities between September 1985 and February 2011. They found 204,561 nuclear medicine reports, which contained information on names and administered dose of radiopharmaceuticals used in procedures.
Ikuta and colleagues then randomly selected 5,000 nuclear medicine reports from the 26-year study period to validate the data extraction process, focusing on the unit of radioactivity, the quantity of administered radiopharmaceutical, and the radiopharmaceutical itself.
The study also manually validated the toolkit's performance on 150 reports to review a single radiopharmaceutical with single administration, a single radiopharmaceutical with multiple administrations, and multiple radiopharmaceuticals with multiple administrations.
PARSE capabilities
The PARSE toolkit, among its functions, converted the nuclear medicine reports into a standardized format for information, such as millicuries and microcuries, to determine radiopharmaceutical quantities.
Ikuta and colleagues also reviewed FDG activity during the past 11 years, with an eye on six months before and after April 2010, when the facility changed its administration protocol. Administered activities greater than 25 mCi were manually reviewed. The distribution of administered activity for iodine-131 sodium iodide from the entire institutional report archive also was examined.
The study sample included 2,359 reports from the final validation; 228 reports (10%) did not contain complete data and were subsequently excluded from the final study.
The researchers measured the performance of PARSE in terms of recall, or the proportion of complete reports from which all data fields were correctly extracted, a measurement roughly analogous to sensitivity, the authors wrote. They also assessed the tool's precision, or the proportion of reports with extracted data that is correct, which they said is similar to positive predictive value.
In the end, the toolkit achieved a combined recall of 97.6% ± 0.7 (2,054 of 2,104) and precision of 98.7% ± 0.5 (2,054 of 2,081). Individual radiopharmaceutical administration scenario statistics all exceeded 97%.
Processing time
Using a standard desktop computer, the data processing took 10 minutes and 57 seconds to pull up the entire 26-year archive of 204,561 reports, including output to the data repository.
The toolkit was able to extract information on 17,562 FDG administrations during the previous 11 years, which included 1,264 administrations in the six months prior to the April 2010 protocol change and 1,241 administrations in the six months after the change.
As for patient-specific radiation dose monitoring, PARSE successfully extracted information on how much of a radiopharmaceutical was given to a 57-year-old patient with stage IV breast cancer to calculate radiation dosimetry.
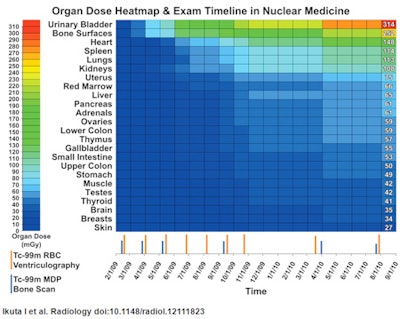 |
| Patient-specific examination timeline and cumulative organ dose heatmap. The chart shows a range with the highest organ doses at the top to identify organs receiving the greatest radiation exposure. Image courtesy of Radiology. |
As the researchers explained, the reason for such patient-specific information is that while organ doses are "relatively higher for areas targeted for repeated imaging (bone surfaces because of bone scans, heart wall because of ventriculography), other bystander organs receive substantial doses due to the biodistribution and pharmacokinetics of the radiopharmaceuticals."
Ikuta and colleagues cited a few limitations of the study, including the single-institution nature of the retrospective research. They also noted that the toolkit uses a "trained array" of radiopharmaceuticals, which may require users to customize the technology to their particular institution.
"While further customization of the programming code may incrementally improve recall and precision, some data extraction errors are unavoidable because of the dictation of extra units of radioactivity, misspelled radiopharmaceuticals, and other typographical errors," the authors wrote.