Multiparametric prostate MRI exams can be difficult to interpret, particularly for those who aren't expert genitourinary radiologists. Machine-learning software may be able to make that task a lot easier, however, according to a proof-of-concept study from the University of California, Los Angeles (UCLA).
A UCLA research team found that a machine-learning algorithm could perform "on par" with the level of accuracy reported in the literature for subspecialized genitourinary radiologists after training and testing the software on a small set of cases that included multiparametric MRI scans and corresponding whole-mount histopathology slides.
"It could enable and assist general or novice radiologists or urologists to really increase their standard of care," Dr. Nelly Tan said.
She shared the group's initial experience with the algorithm during a presentation at the recent annual meeting of the Society of Imaging Informatics in Medicine (SIIM 2016) in Portland, OR.
Common cause of cancer death
Prostate cancer is the second leading cause of cancer death in men in the U.S. The most important prognosticator for clinical outcomes is the Gleason score, which measures cancer aggressiveness on biopsied tissue samples. However, the current standard of care -- transrectal ultrasound-guided biopsy -- has a very low risk stratification predictive value of 15% to 30%, Tan said.
 Dr. Nelly Tan of the University of California, Los Angeles.
Dr. Nelly Tan of the University of California, Los Angeles."As a result, over the last four to six years, multiparametric MRI of the prostate has caused a paradigm shift in how prostate cancer is diagnosed," she said.
Recently, the 2016 consensus statement from the American Urological Association and the Society of Abdominal Radiology noted that MRI is indicated in patients with a prior negative biopsy. However, prostate MRI is difficult; it requires subspecialized training to interpret, is time-consuming, and is characterized by high interreader variability, Tan said.
Other research groups have developed machine-learning algorithms for prostate MRI, but those efforts have been limited to the peripheral zone of the prostate -- where most prostate cancer develops, she said. However, 30% of prostate cancer cases develop in the transitional zone of the prostate.
In addition, these groups have typically used biopsy as a reference standard for training, rather than surgical pathology. That can lead to issues with classification error, she said.
"Also, only a few approaches have integrated regional information between the [multiple MRI] parameters," Tan said.
A predictive map
As a result, the researchers developed a supervised machine-learning algorithm covering the whole prostate that would utilize regional MRI features and be trained on whole-mount histopathology as the reference standard. The goal was to provide a simplified, predictive map of prostate cancer, helping to improve diagnostic accuracy, reduce reader variability, and accelerate reading time, Tan said.
They then trained the algorithm to recognize potential cancer in a retrospective study in which they collected clinical, biopsy, MRI, and surgical pathology information from seven prostate cancer patients. The patients had a mean prostate volume of 27.2 cc (standard deviation [SD] = 9.5) and a mean tumor diameter on MRI of 1.7 cm (SD = 0.7).
On preoperative multiparametric MRI, the Prostate Imaging Reporting and Data System (PI-RADS) score was 4/5 in four cases and 5/5 in three cases. The tumors were staged as American Joint Committee on Cancer (AJCC) stage T2 in three cases and T3 in four cases. Postoperative histopathology showed a Gleason score of 3+4 in four cases, 4+3 in two patients, and 4+5 in one patient.
Each patient's MR images -- including T2, high b-value diffusion-weighted images, and apparent diffusion coefficient maps -- as well as corresponding whole-mount histopathology slides, were contoured, or manually segmented, using the OsiriX image processing software.
The pathology images were then coregistered on the MR images and input into the UCLA group's algorithm, which employed kernel spectral analysis to train on the dataset. As a result, the software can provide simplified predictions of where tumors are located based on the imaging features in the region of interest, according to the group.
To test the performance of the algorithm for detecting new cases of prostate cancer, the researchers utilized a model validation technique called leave-one-out cross-validation. Applied to the same dataset used for training, the machine-learning algorithm produced an average accuracy of 71% ± 8% in identifying the cancerous region on the images, according to the group.
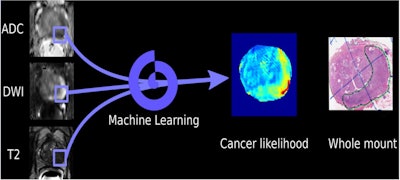 Schematic of machine-learning algorithm for detecting prostate cancer on multiparametric MRI. Image courtesy of Dr. Nelly Tan.
Schematic of machine-learning algorithm for detecting prostate cancer on multiparametric MRI. Image courtesy of Dr. Nelly Tan."The trained algorithm performed as well as a [genitourinary] subspecialized radiologist," Tan said.
Research in the literature has shown that subspecialized genitourinary radiologists have an average accuracy of about 68%, while general radiologists have an average accuracy of approximately 30% to 40%, she said.
Tan acknowledged a number of limitations of the study, including the use of a small training set for the algorithm. They also did not evaluate cases with low-grade Gleason scores and have not yet incorporated dynamic contrast-enhanced MRI images. In addition, they applied a single algorithm to the same testing dataset and performed manual prostate segmentation, she said.
The researchers are now continuing to develop the algorithm, including validating it on a larger dataset and then evaluating its segmentation performance, Tan said. They will then seek to optimize the algorithm and assess whether it can help radiology residents and fellows. If all goes well, the team plans to implement the algorithm in a prospective study, she said.