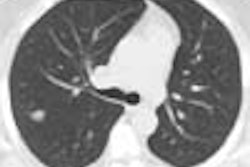
Monitor manufacturer BarcoView of Kortrijk, Belgium, has received U.S. Food and Drug Administration approval for an enterprise-wide image-quality-management software package. The application, MediCal Administrator, manages the soft-copy quality of an institution’s complete installed base of display monitors.
The software package generates customized reports of each display system’s performance and sends automatic alerts if a display system does not meet the quality standards set. It also permits system administrators to schedule display maintenance.
By AuntMinnie.com staff writersOctober 18, 2002
Related Reading
BarcoView builds family of flat-panel monitors, September 10, 2002
BarcoView joins European soft-copy review trial, March 3, 2002
BarcoView gets clearance for Coronis, February 14, 2002
BarcoView gets a Dell deal, dude, January 28, 2002
BarcoView inks monitor supply deal with Agfa, January 18, 2002
Copyright © 2002 AuntMinnie.com