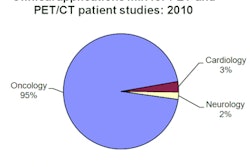
The U.S. Food and Drug Administration (FDA) has proposed new guidelines for high-quality clinical studies and a draft guidance to clarify how benefit-risk determinations are made during premarket approval (PMA) review of certain medical devices.
The benefit-risk guidelines target PMA applications with recommendations to improve the predictability, consistency, and transparency of the premarket review process for applicable devices. The goal is to help manufacturers navigate the approval process more easily.
For example, the communiqué proposes that medical device reviewers use a worksheet to document how they make benefit-risk determinations. In certain cases, this document could be made public postapproval, making the FDA's decision-making process more transparent.
The FDA is also seeking to help researchers and manufacturers design better quality clinical studies in support of PMA applications for medical devices.
The proposed guidance outlines agency expectations for clinical trial design for class III devices, such as minimizing data bias and variability, setting appropriate study objectives, selecting the appropriate type of study, and choosing study sites and study participants.