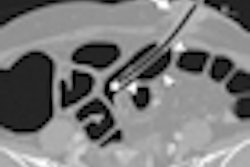
Interventional technology developer Cook Medical has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Evolution duodenal controlled-release stent.
To provide relief to patients with advanced small intestine cancer, the stent is designed to improve quality of life for those experiencing issues with malignant gastric outlet obstruction (GOO), according to the company. GOO is a late-stage complication of a variety of gastrointestinal-related cancers.
The Evolution duodenal stent features 18 crowns at both the distal and proximal ends, which allow the stent to adapt to the natural curvature of the anatomy, Cook said.