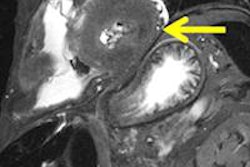
NinePoint Medical said it has received an additional 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its NvisionVLE optical coherence tomography (OCT) imaging system.
The clearance expands the indication for NvisionVLE to include the imaging of esophageal tissue microstructure, NinePoint said.
NvisionVLE generates a volumetric image of the esophageal tissue in real-time that can be viewed simultaneously in cross section and longitudinally. The cross-sectional scan is 6 cm in length and 3-mm deep, permitting visualization of multiple tissue layers, the company said.