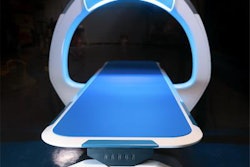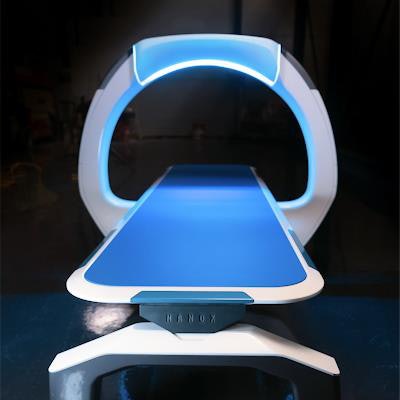
X-ray developer Nanox said it has applied for 510(k) clearance with the U.S. Food and Drug Administration (FDA) for the first version of the company's multisource 3D digital tomosynthesis x-ray system.
The company positioned the filing as a major achievement in its quest to bring its multisource Nanox.ARC system to market. Nanox in April announced clearance of a version of Nanox.ARC that uses a single x-ray source.
The multisource version of Nanox.ARC is a more sophisticated version of the single-source system, with multiple x-ray sources arrayed around the patient to enable 3D tomosynthesis acquisitions. Nanox is positioning its systems as cost-effective products that could make medical imaging more accessible in developing parts of the world.