The U.S. Food and Drug Administration (FDA) has asked Israeli x-ray developer Nanox for additional information regarding the regulatory submission for its Nanox.ARC multisource x-ray system to correct what the agency said were "deficiencies" in the submission.
In an August 12 filing with the U.S. Securities and Exchange Commission (SEC), Nanox said that the FDA had placed a hold on the submission "pending a complete response to the FDA's list of deficiencies" with the filing. Nanox has 180 days to respond to the FDA request.
Nanox earlier this year submitted paperwork for 510(k) clearance of Nanox.ARC, a digital radiography system that employs multiple x-ray sources rather than a single source that's typically used on most x-ray devices. Nanox already has FDA clearance for a single-source version of Nanox.ARC.
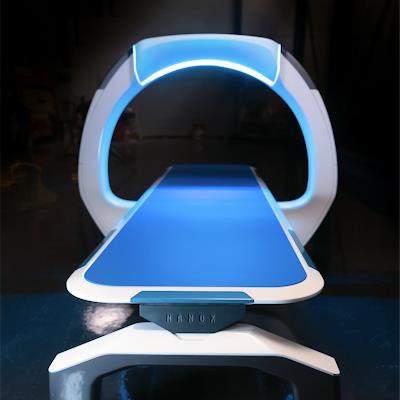
 The Nanox.ARC digital x-ray system. Image courtesy of Nanox.
The Nanox.ARC digital x-ray system. Image courtesy of Nanox.The multisource version of Nanox.ARC will be capable of performing tomosynthesis studies on patients, and it is key to the company's vision of installing low-cost x-ray systems in developing areas of the world. The company earlier this month also announced its plans to acquire artificial intelligence developer Zebra Medical Vision and teleradiology specialist USARad Holdings to add an image interpretation capability to its offerings.
In the SEC filing, Nanox did not indicate the nature of the deficiencies mentioned in the FDA notice. However, it did state that it planned to "continue to optimize and develop" new features for the multisource version of Nanox.ARC, and the company may use the FDA's feedback as part of a new 510(k) regulatory submission for the next version of the system to the FDA in the fourth quarter of 2021.
Nanox followed a similar path to regulatory clearance for the single-source version of Nanox.ARC, with the FDA asking for additional data on the submission before eventually clearing the system earlier this year.