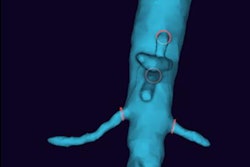
The U.S. Food and Drug Administration (FDA) has granted de novo clearance for Philips Healthcare's IVC (inferior vena cava) filter removal laser sheath to remove filters when other methods fail.
CavaClear, which was granted a breakthrough device designation from the FDA, has been shown to have a success rate of more than 99%, with low complication rates, according to the company.
IVC filters are used to treat patients with venous thromboembolism, however, they can fracture and travel through the bloodstream to other parts of the body, Philips said.