Jan Makela.
Jan Makela.
In the U.S., more than 6 million Americans have Alzheimer's disease, a debilitating and far-reaching disease that accounts for 60% to 80% of dementia cases, and by 2050, this number is projected to rise to nearly 13 million, according to Alzheimer’s Disease International.
Alzheimer’s has been a formidable challenge for our society, patients, caregivers, and healthcare systems for decades. The disease affects memory, thinking, and behavior. Its symptoms typically start with mild cognitive impairment and eventually grow severe enough to interfere with daily tasks.
Historically, medications and therapies have been of limited effectiveness and have focused on treating the symptoms of the disease rather than addressing its root cause. This lack of effective treatment, coupled with late-stage diagnosis, has resulted in a lack of hope and few options for patients and their families. Additionally, an increasing aging population has further exacerbated the burden of this disease on healthcare systems.
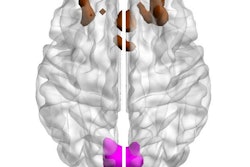
A hallmark of Alzheimer's Disease is the build-up of toxic proteins in the brain. One of these proteins, beta-amyloid, clumps together to form plaques, causing brain cells to die. With the emergence of new therapies, including U.S. Food and Drug Administration (FDA) approval of a medication that targets amyloid protein in the brain, we have reason to be optimistic. The FDA approval of this new therapy is an important development for clinicians who will now have greater access to a new drug that was developed to help limit the progress of a disease that currently has no cure.
The promise of imaging
One challenge is that advances in Alzheimer’s care can only help people if they are reaching the right patients at the right time in their patient journey. This can only be accomplished with a combination of innovative diagnostic, treatment, and monitoring technology that can enable timely interventions and personalized treatment plans.
This is especially true because new Alzheimer’s therapies can only be prescribed to patients with mild cognitive impairment or mild dementia. Eligibility also requires the confirmation of amyloid in a patient’s brain. Amyloid PET imaging is the only noninvasive method that can directly visualize the amount and regional extent of brain amyloid, enabling clinicians to distinguish Alzheimer's disease from other causes of dementia or memory loss and help ensure appropriate medical care and treatment.
Ultimately, with this new Alzheimer’s treatment option, and utilization of accurate imaging technology, clinicians can ensure effective diagnostic and monitoring capabilities for people with Alzheimer’s, which could help improve patient outcomes. More accurate diagnostic and monitoring tools can also help manage the high cost of new therapies by preventing the misuse of expensive treatments.
Reimbursement barriers
Unfortunately, until recently, there were significant barriers to receiving amyloid PET imaging in some regions. The U.S. Centers for Medicare and Medicaid Services (CMS), for example, did not cover amyloid PET scans outside of clinical trials for Medicare beneficiaries and only covered one scan per patient’s lifetime. In October 2023, however, a significant step forward occurred when CMS expanded coverage of amyloid PET imaging for the diagnosis of Alzheimer’s Disease.
The new Medicare policy provides local Medicare administrators with the ability to cover PET scans (beyond clinical trials) and without a limitation of one scan within a patient’s lifetime. Under this new policy, a valuable Alzheimer’s Disease diagnostic tool will now be more accessible to Medicare beneficiaries across the country because of this policy change.
Beyond coverage, improved payment policies can help increase access to PET scans as well. For the hospital outpatient setting, Medicare currently packages the payment for radiopharmaceuticals into an overall payment for groupings of nuclear medicine procedures including PET.
This has led to inadequate payment for precision radiopharmaceuticals and challenges for some providers seeking to offer these imaging services. Medicare acknowledged this issue during the Calendar Year 2024 Hospital Outpatient rulemaking process and over 130 stakeholders responded to Medicare’s call for comment with the vast majority supporting separate payment for the radiopharmaceutical. Stakeholders will continue to work with the agency so that hospital outpatient facilities are adequately reimbursed in the future.
Additionally, 2023 saw activity in Congress on the topic, with reintroduction of the Facilitating Innovative Nuclear Diagnostics Act, or FIND Act, in the House and Senate. The bill is intended to improve hospital outpatient reimbursement for precision nuclear scans for Alzheimer’s and other diseases. The FIND Act is bipartisan, budget-neutral legislation to solving access issues and was included in a House hearing on Medicare’s payment policies.
Therapy planning, continuous monitoring
Imaging technology is critical for people with Alzheimer’s because it aids clinicians in characterizing the disease at an individual level to allow for the creation and implementation of tailored treatment strategies. Amyloid PET imaging is expected to help clinicians in the application of amyloid-targeting therapies.
Meanwhile, MR imaging is considered the gold standard for imaging the potential side effects of amyloid-targeting therapeutics, including swelling and bleeding in the brain. MR scanning is included in the recent FDA-approved amyloid targeting therapy instructions for use, to ensure patients on these therapies are periodically scanned with MR or PET/MR before and during treatment.
PET/MR imaging can help healthcare professionals to confirm the patient’s amyloid status while at the same time helping to visualize fine details of the entire brain structure in a single scan.
Imaging technology can also enable the ongoing assessment of disease progression and treatment effectiveness. Amyloid PET imaging is currently the only non-invasive method to monitor and confirm amyloid reduction for patients undergoing therapy.
The future
It is an important moment in time for people with Alzheimer’s and their loved ones, as these new treatment options instill hope in managing a challenging disease that has no cure. While approvals are currently happening regionally and nationally, this new therapy has the potential to benefit millions of patients worldwide each year. By integrating advanced imaging technologies into Alzheimer’s care, we can substantially improve diagnosis, therapy planning and delivery, and monitoring. With this, we are seeking to achieve something historic — helping to improve patient outcomes, reduce caregiver burden, and enhance overall healthcare efficiency in the face of a disease that for so long has proven intractable.
The potential of imaging technology in Alzheimer’s care is nothing less than transformative. To realize this potential, we must be committed to continued investment in research and technology and advocate for healthcare policies that will benefit patients and providers.
Jan Makela is the president and CEO of GE HealthCare Imaging, which offers technologies and services across MR, CT, PET, nuclear medicine, x-ray, women’s health, interventional, surgery, imaging analytics, and software. Jan previously served as president and CEO of GE HealthCare Global Services from 2017 to early 2020, where he oversaw the global development and execution of GE HealthCare’s service solutions and operations around the globe. Jan has over 20 years of industrial experience and earned a master’s degree in engineering from the University of Cambridge.
The comments and observations expressed are those of the author and do not necessarily reflect the opinions of AuntMinnie.com.