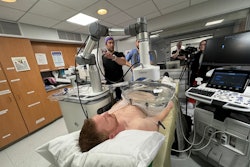
Since the U.S. Food and Drug Administration's (FDA) approvals of radiopharmaceuticals for neuroendocrine tumors and then for prostate cancer, theranostics has picked up momentum in clinical practice, propelled by encouraging research.
Theranostics pairs diagnostic biomarkers that can be visualized on nuclear medicine imaging with therapeutic agents that share a specific target in diseased cells or tissues. After the therapeutic agent binds to the cancer cells, the tumors are treated in such a way that aims to prevent collateral damage to healthy cells and improve overall outcomes.
Theranostics isn’t new; nuclear medicine departments have provided thyroid cancer treatment with radioactive iodine (I-131) since the first half of the 20th century. Today, however, a new pillar of cancer treatment has emerged using the radionuclides gallium-68 (Ga-68) and lutetium-177 (Lu-177), and it appears to be advancing rapidly. Theranostics could be poised to become an accepted first-line therapy for some cancers.
Log in to view the full article
Since the U.S. Food and Drug Administration's (FDA) approvals of radiopharmaceuticals for neuroendocrine tumors and then for prostate cancer, theranostics has picked up momentum in clinical practice, propelled by encouraging research.
Theranostics pairs diagnostic biomarkers that can be visualized on nuclear medicine imaging with therapeutic agents that share a specific target in diseased cells or tissues. After the therapeutic agent binds to the cancer cells, the tumors are treated in such a way that aims to prevent collateral damage to healthy cells and improve overall outcomes.
Theranostics isn’t new; nuclear medicine departments have provided thyroid cancer treatment with radioactive iodine (I-131) since the first half of the 20th century. Today, however, a new pillar of cancer treatment has emerged using the radionuclides gallium-68 (Ga-68) and lutetium-177 (Lu-177), and it appears to be advancing rapidly. Theranostics could be poised to become an accepted first-line therapy for some cancers.
Theranostics provides doctors a framework for true "precision medicine," said Penny Vroman, MD, a dual board-certified nuclear radiologist and an associate professor at University of Texas (UT) Health Science Center in San Antonio, where Vroman played a key role in establishing the growing theranostics center.
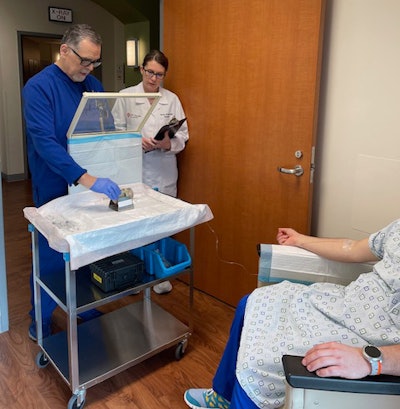 Team members from the theranostics clinic under University of Texas Health Science Center at San Antonio's Department of Radiology show one aspect of a Pluvicto infusion treatment. The L block lead shield over the syringe helps protect certified nuclear medicine technologist James Johnson (left), and nuclear radiologist Penny Vroman, MD (right), from radiation emitted by the drug. (Photo courtesy of UT Health San Antonio Department of Radiology)
Team members from the theranostics clinic under University of Texas Health Science Center at San Antonio's Department of Radiology show one aspect of a Pluvicto infusion treatment. The L block lead shield over the syringe helps protect certified nuclear medicine technologist James Johnson (left), and nuclear radiologist Penny Vroman, MD (right), from radiation emitted by the drug. (Photo courtesy of UT Health San Antonio Department of Radiology)
"Until about 2013, only a few radiopharmaceutical agents were used to help treat cancer patients," Vroman explained in an interview with AuntMinnie.com. "Since 2013 we've had several more radiopharmaceutical therapies get FDA approval. The field has really started to rapidly expand, and we expect that trajectory just to continue. Within that are two trial results that just came out recommending first-line therapy with a theranostic agent, which is the first time a theranostic agent has ever been recommended as first-line therapy."
Neuroendocrine tumor treatment
The rise of theranostics begins with Lutathera (lutetium Lu-177 DOTATATE), a radioactive, radioligand therapy from Novartis for the treatment of somatostatin receptor-positive neuroendocrine tumors (NETs). This theranostic therapy first demonstrated how using diagnostic imaging with a radioactive probe, gallium-68 (Ga-68 dotatate injection), could locate NETs on PET scans. After binding to the receptor, the drug works by entering the cell allowing radiation to cause damage to the tumor cells.
Lutathera was approved for adults by the FDA in 2018 after the therapy resulted in progression-free survival (median overall survival of 11.7 months) when used in combination with standard-dose octreotide in the treatment of metastatic, locally advanced, inoperable somatostatin receptor-positive NETs. In these patients, treatment with high-dose octreotide had been shown to worsen their tumors.
UT's Vroman said she is preparing for more to come out of the results of a phase III trial (NETTER-2) with Lutathera. Earlier this month, Cancer.gov news reported a new development. Lutathera in combination with octreotide appeared to work better in patients who had not yet been treated with octreotide. The research suggested the combination might be beneficial as an initial therapy for those with advanced neuroendocrine tumors.
Jaydira del Rivero, MD, of the National Cancer Institute's Center for Cancer Research, wrote that the NETTER-2 study results are “groundbreaking” and that giving Lu-177 DOTATATE as an initial treatment for these cancers could be "practice changing.”
Traditional treatments didn't slow the disease in these patients, noted UT’s Vroman. In contrast with the previous NETTER-1 trial, NETTER-2 combined Lutathera with the traditional therapy and then compared outcomes to traditional therapy alone. Researchers found that the combination extended a patient's progression-free survival by three times longer than traditional treatment alone, Vroman said.
"The recently published results were just phenomenal, outstanding," she told AuntMinnie.com.
Prostate cancer treatment
Another Lu 177-based radioligand therapy called Pluvicto (Lu-177 vipivotide tetraxetan) from Advanced Accelerator Applications USA, a Novartis company, is also accelerating theranostics. This drug relies on a gallium Ga-68, too, as a diagnostic imaging agent. The theranostic pair designed for metastatic castration-resistant prostate cancer (mCRPC) reached FDA approval together in 2022, following the successful phase III VISION trial conducted Canada, Europe, and the U.S.
A breakthrough with mCRPC is based on particularly expressive characteristics of the prostate-specific membrane antigen (PSMA) biomarker observed with prostate cancer cells. For mCRPC, the approach uses the targeting compound (PSMA-617) that binds to prostate cancer cells and with Lu-177 inhibits tumor growth.
Moving forward, UT's Vroman is watching for results to come of PSMAfore, one of many clinical trials still revolving around Lu-177. This multiyear, phase 3 study is further evaluating the impact of Lu-177 PSMA-617 where it is considered appropriate to delay taxane-based chemotherapy, to see if it improves overall survival in participants with progressive PSMA-positive mCRPC.
If favorable results bear out, the outcomes of PSMAfore could be a significant development in prostate cancer treatment, according to Vroman.
Theranostics in practice
UT Health San Antonio is one of many institutions in the U.S. with a growing theranostics practice. Having already established the drug Lutathera for NETs, UT Health began offering Pluvicto for prostate cancer in August 2023 to a small number of qualifying, eligible patients.
Vroman played a key role in establishing the large, multidisciplinary theranostics practice -- what she describes as a "cohesive" team that provides patient-centered care after a patient has been diagnosed with cancer. The nuclear radiologist, nuclear medicine technologist, and radiation safety team are all present to administer an infusion.
"It's full-spectrum, personalized care," Vroman told AuntMinnie.com.
Vroman's experience dates back over a decade to her service in the U.S. Air Force where she achieved the rank of lieutenant colonel and served as nuclear medicine department chief at Brooke Army Medical Center at Joint Base San Antonio-Fort Sam Houston. Vroman helped start the theranostics program there with Lutathera.
For prostate cancer treatment using Pluvicto, UT Health San Antonio started with five patients who were referred by oncologists from the Mays Cancer Center, home to UT Health San Antonio MD Anderson Cancer Center. The current pilot therapy program has recently been expanded to 10 patients, and Vroman anticipates opening the therapy to the community this year.
Moreover, based on the NETTER-2 trial results, Vroman said that she expects UT Health to begin offering Lutathera to the community later this year.
An energized field
Excitement over the value of theranostics was evident at the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting, where a new theranostics pair earned Image of the Year honors. The SNMMI and other organizations are responding to burgeoning interest, said Hossein Jadvar, MD, PhD, a professor of radiology, urology, and biomedical engineering at Keck School of Medicine at the University of Southern California (USC) in Los Angeles.
Jadvar was among those who led a special SNMMI theranostics track at SNMMI's mid-winter meeting this past February. Jadvar also serves as president of the SNMMI Therapy Center of Excellence amid other responsibilities with the U.S. Nuclear Regulatory Commission Advisory Committee on Medical Uses of Isotopes (NRC-ACMUI) and USC's Norris Comprehensive Cancer Center.
"There's a lot of interest where people don't have this in their hospital or clinic and they want to know how to set it up," Jadvar said in an interview with AuntMinnie.com. SNMMI's course included business planning, daily operation, dosimetry, alpha-therapy, and sessions on the cancers for which theranostics is used most often: neuroendocrine, thyroid, prostate, and emerging in pediatric neuroblastoma.
"These meetings are very much geared toward the people who are actually doing theranostics," Jadvar continued.
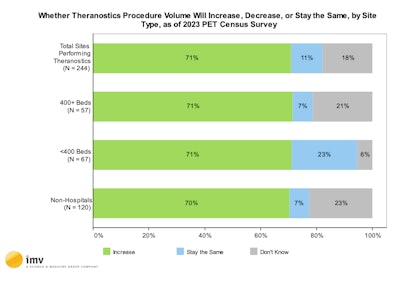 (Source: 2024 IMV PET Market Summary Report)
(Source: 2024 IMV PET Market Summary Report)
The event also covered new theranostic targets and combination therapy. In addition, medical oncologists, radiation oncologists, surgical oncologists, pathologists, radiologists, nuclear medicine physicians, and geneticists participated in mock tumor boards for prostate and neuroendocrine tumors.
"Theranostics centers, the establishment of the whole field became energized after these new agents were approved, first with Lutathera for neuroendocrine tumors and then with Pluvicto for prostate cancer, and the list will grow," Jadvar said.
Stay tuned for part 2 of our series on theranostics.